

A conversation on why covalence is moving from last resort to first-line strategy in small molecule discovery.
As part of our mission to tackle some of the toughest targets in drug discovery, we’ve recently expanded our early discovery capabilities to include covalent compound library screening. Our early discovery site in Leiden is already well-known for its success in first-in-class and hard-to-drug targets, and this addition reflects a growing shift in the field: covalent approaches are no longer a last resort, they are becoming a smart, mechanism-led starting point for small molecule discovery.
To understand what makes covalent screening so powerful (and what it takes to get it right) we spoke with screening expert and Leiden leader Gregg Siegal. In this Q&A, Gregg shares his perspective on why covalent drugs are in the spotlight and what it takes to perform meaningful hit discovery. We also dig into how to build assays that can unpick the data generated by these high-potential, high-complexity compounds, and why selectivity, structural insight, and kinetics are critical for turning raw reactivity into real therapeutic potential.

Q: Gregg, why are some biotechs pivoting to covalent compounds, and why now?
GS: For a long time, covalent compounds carried this stigma that they were too risky, especially from a tox perspective. People thought they’d react with everything in sight and just blow up in the clinic. But that’s been debunked. We’re well past that.
You’ve got 128 covalent drugs approved by the FDA as of last year1. The first two KRAS drugs that made it through? Covalent. Nobody seriously argues anymore that covalent equals dangerous. That mindset’s gone. What’s changed is that the benefits are now obvious, and the tools to do it right are finally in place.
Q: What’s the actual advantage of going covalent?
GS: It’s your hook. Covalent compounds give you a chemical handle to grab onto a target, even if you don’t have a defined pocket.
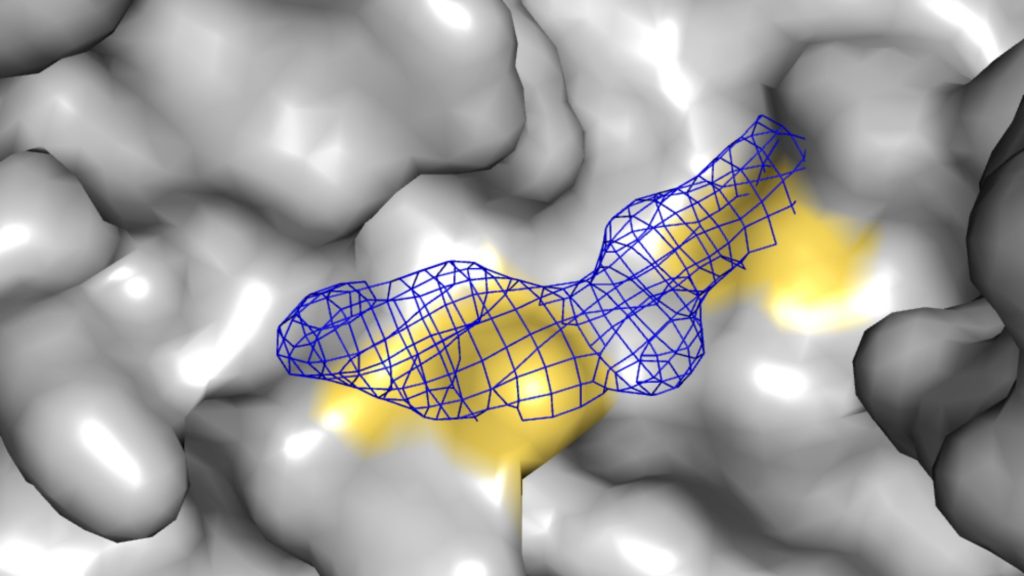
This matters especially for PPI targets, where the surfaces are often flat, flexible, and dynamic. Sometimes pockets only show up 2–3% of the time. With fragments or traditional libraries, that’s a nightmare. But a covalent compound doesn’t need the pocket to stay open, it just needs one shot. One good nucleophile, and you’re in.
Q: What’s the catch? What makes covalent screening hard to do well?
GS: Getting a hit isn’t hard. Getting the right hit is where it gets tricky.
Covalent libraries are full of reactive compounds. Some will react with any residue they encounter. If you’re not careful, you’ll just denature the protein, or trigger aggregation. So, after the screen, you’ve got a pile of raw hits. Some are good, many are not.
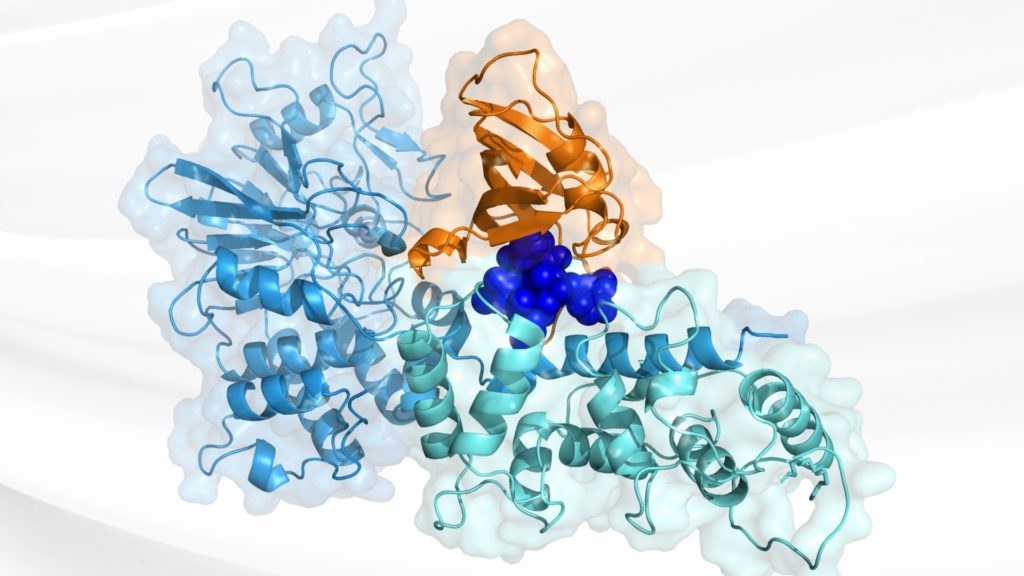
You need to dissect the mechanisms. Are they binding where you want? Are they clean? We run mass spec to make sure you’re getting one or two adducts per protein, not five. We follow that with crystallography to confirm the binding mode. And then we run kinetic profiling to measure kinact and KI. That’s where the expertise matters.
Q: How do you set up the screen to make sure it’s biologically relevant?
GS: We build an assay that reports directly on the biological activity the client wants to see, which these days, is often disruption of a protein–protein interaction. In a recent study, for example, we used a fluorescence polarization assay that showed whether our transcriptional regulator was bound or free.
We screened 11,000 compounds and looked for a reduction in binding. That tells us a compound blocks binding of the co-transcriptional regulator and not just that it’s reactive. From there, we triage. And that’s where the power of structure comes in. You want to go from raw hits to validated starting points, and you want to do it quickly. We had structures of 7 different hits in a month.
Q: Who should be looking at covalent screening?
GS: Anyone working on “unligandable” targets. If we’re honest, that’s a rapidly growing portion of drug developers because the targets we, as an industry, are working with are becoming more and more challenging. Oncology, transcription factors, scaffolding proteins, immune signaling, molecular glues; this is where traditional small molecule approaches might struggle unless you innovate.
If you’re working with difficult targets, stalled programs, or exploring molecular glues, covalent could be a really smart move. Especially if you’re short on time and need a progressable, structurally enabled hit to start a campaign.
Q: How can researchers learn more about initiating covalent screening?
GS: It sounds clichéd, but the most straightforward thing to do is send us a message and talk to us. This is what we do, this is what we’re passionate about. We’ll sit down with you and listen. We’ll ask questions and work to understand what you need and what you’re aiming to do. Then our highly experienced early discovery team will work out how we can best help you get to where you need to go. Once you have all the information and a plan, it’s over to you to make a decision on whether to move forward.
You can explore covalent library screening at the Oncodesign-ZoBio Group by downloading our guide. Alternatively, get in touch with us for an informal conversation about whether covalent screening might be a viable approach for your tough target.
Dr. Gregg Siegal obtained his Ph.D. in biochemistry at the University of Rochester in the USA. During subsequent post-doctoral research with Professor Kurt Wüthrich at the ETH in Switzerland and Professor Paul Driscoll at the Ludwig Institute of Cancer Research in the UK, Dr. Siegal developed expertise in protein NMR and its applications in modern drug discovery. He moved to Leiden University in 1997 where he received a Dutch Royal Society Fellowship to form his own research group. During this period, he developed the Target-immobilized NMR (TINS) ligand screening technology which became the basis of ZoBio, spun out in 2004 and specializing in complex small molecule early discovery research and hit identification.
The ZoBio team in Leiden, Netherlands, joined the Oncodesign Services group in early 2024, adding their extensive expertise and experience in tactical early discovery approaches to our existing late discovery and preclinical portfolio.
References
- Dalton, S. E., Pietro, O. D. & Hennessy, E. A Medicinal Chemistry Perspective on FDA-Approved Small Molecule Drugs with a Covalent Mechanism of Action. J. Med. Chem. 68, 2307–2313 (2025).
Want to discuss covalent compound screening with our team? Contact us: