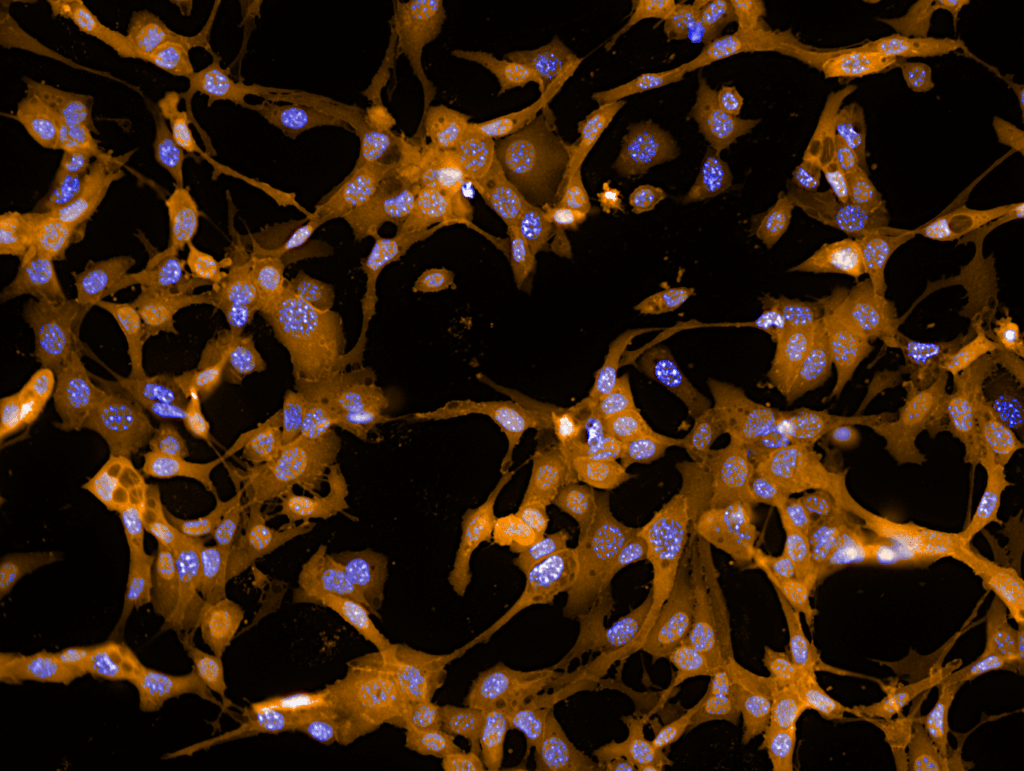


Don’t guess. Ask the cell. Reporter cell assays make it possible.
Author: Dr. Nicolas Ancellin, Senior Program Director & Site Director, Oncodesign Services
Let’s dive into the wild, wonderful world of engineered cells in drug discovery-specifically, those cellular superheroes equipped with reporter cell assays.
Drug discovery is a bit like dating in the modern age: you’ve got millions of candidates (compounds), and you’re swiping left or right, hoping to find “the one” that checks all the right boxes. But instead of awkward coffee dates, we’ve got engineered cells offering precision and power that traditional discovery methods can only dream of. These cells aren’t just sitting there looking pretty, they’re genetically tweaked to act as living, breathing detectors, flashing signals when a compound interacts. Whether it’s a reporter cell assay lighting up like a Christmas tree or a pathway study revealing the inner workings of a cell, these tools are revolutionizing how we find new drugs.
The glow-up drug developers have been waiting for…
 So, what’s the big deal with reporter cell assays? Imagine you’re trying to figure out if a compound is an inhibitor, agonist, or antagonist – is it a friend, a foe, or just flirting with your target protein? Reporter cell assays are your wingman. By applying a glowing tag (think fluorescence or luminescence) onto a specific gene or pathway, you can watch in real time as the cell reacts to a drug. It’s like giving the cell a megaphone to shout, “Hey, this compound is messing with my NFkB pathway!” or “Whoa, this one’s cranking up my TCF promoter!” These assays are so precise, they can serve as primary screens, lead optimization tools, or even the star of a phenotypic screen where you’re fishing for a vibe rather than a specific target.
So, what’s the big deal with reporter cell assays? Imagine you’re trying to figure out if a compound is an inhibitor, agonist, or antagonist – is it a friend, a foe, or just flirting with your target protein? Reporter cell assays are your wingman. By applying a glowing tag (think fluorescence or luminescence) onto a specific gene or pathway, you can watch in real time as the cell reacts to a drug. It’s like giving the cell a megaphone to shout, “Hey, this compound is messing with my NFkB pathway!” or “Whoa, this one’s cranking up my TCF promoter!” These assays are so precise, they can serve as primary screens, lead optimization tools, or even the star of a phenotypic screen where you’re fishing for a vibe rather than a specific target.
Now, let’s add some molecular biology magic. Building these cellular constructs is where the fun really begins – like playing with biological Legos, but instead of stepping on them in the dark, you’re creating masterpieces. Want a fusion protein that glows when your pathway is activated? Done. How about a double reporter system to double-check your results? Easy. Maybe you’re feeling fancy and want to monitor secretion or degradation, for example: watching a protein get chewed up by a PROTAC. The possibilities are endless, and the readouts? Oh, they’re a feast for the senses: fluorescence for the visually inclined, luminescence for the night owls, or the classic enzymatic assay for those who appreciate a good old-fashioned reaction. It’s like choosing between a fireworks show, a laser display, or a satisfying campfire crackle.
And here’s the kicker: these systems are fast. We’re talking ‘from scratch to validation in less than two months’ fast. That’s quicker than it takes to binge-watch a season of your favorite sci-fi series. You start with an idea, such as a reporter for the NFkB pathway, which is like the cell’s inflammation alarm system, and before you know it, you’ve got a glowing cell line ready to test a library of compounds. Or maybe you’re into the TCF promoter assay, tracking Wnt signaling like a cellular GPS. The list of reporter examples is a veritable alphabet soup: ERE for estrogen response, CRE/CREB for cyclic AMP shenanigans, HRE for hypoxia, XRE for xenobiotic responses, SRE for serum response, p53 for tumor suppression, MYC for cell growth, ISRE for interferon signaling. It’s like a buffet of cellular pathways, and you’re the chef assembling the perfect dish.
Pictured above: Vero E6/TMPRSS2 cell infected with SARS-Cov2 (strain SK-BMC5/2020). Nucleoprotein / NP Antibody (nucleocapsid protein- green) immunofluorescence vs. nucleus staining (blue).
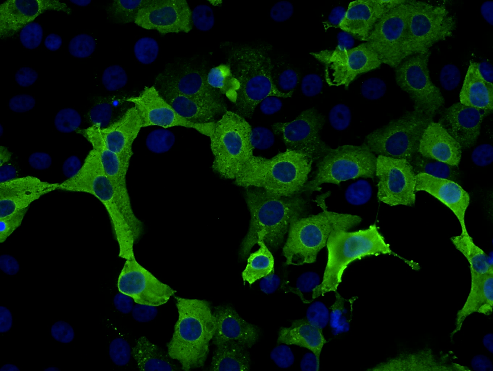
Pictured below: Phenotypic screen using a tagged ‘protein of interest’.
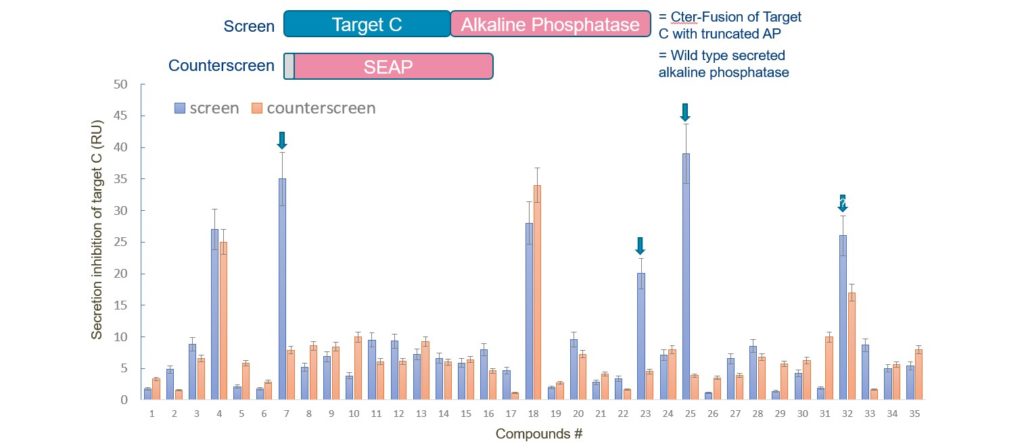
What happens when molecular biology gets creative?
 Let’s zoom in on one of my favorites: the NFAT reporter cell assay. This little gem monitors calcium signaling, which is a big deal for things like immune responses or GPCR (G protein-coupled receptor) activity. Want to make it even cooler? Coexpress a chimeric G protein to flip the signaling switch toward calcium, and suddenly you’ve got a tricked-out system to study GPCRs that wouldn’t normally play in the calcium sandbox. It’s like giving a shy kid the confidence to dance at the prom and then watching them steal the show.
Let’s zoom in on one of my favorites: the NFAT reporter cell assay. This little gem monitors calcium signaling, which is a big deal for things like immune responses or GPCR (G protein-coupled receptor) activity. Want to make it even cooler? Coexpress a chimeric G protein to flip the signaling switch toward calcium, and suddenly you’ve got a tricked-out system to study GPCRs that wouldn’t normally play in the calcium sandbox. It’s like giving a shy kid the confidence to dance at the prom and then watching them steal the show.
But there’s more. Reporter cell assays aren’t just for pathway spying, they’re also MVPs in target engagement assays. Take kinases, the cellular workhorses that phosphorylate everything in sight. A reporter can tell you if your compound is cozying up to the kinase or giving it the cold shoulder. Or consider PROTACs, those crafty molecules that tag proteins for destruction. A degradation-monitoring reporter can show you the moment your target protein gets hauled off to the proteasome.
Now, let’s get a little quirky with frameshift monitoring. Imagine you’re studying a genetic slip-up where the reading frame of a gene goes haywire, kind of like a typo that turns “cat” into “cauliflower.” A reporter cell assay can flag compounds that fix this mess, turning a phenotypic screen into a treasure hunt. This approach has real-world credibility. Take gentamicin-like compounds, discovered through cell assays that monitor readthrough mechanisms, where the ribosome ignores curfew and just keeps on partying. These drugs help override nonsense mutations, and the reporter cell assay was the spotlight that picked them out. Another shining star? Risdiplam, a market-approved drug for spinal muscular atrophy. Its discovery came from a cell assay that caught it boosting SMN protein production, proving that engineered cells can take you from the lab bench to a shelf in the pharmacy.
Then there’s the PCSK9 story: a tale of secretion, reporters, and unexpected twists. PCSK9 is a protein that meddles with cholesterol levels, and researchers wanted to block its exit from the cell. They rigged up a reporter to track PCSK9 secretion, expecting to find a small molecule to slam the door shut. Instead, they stumbled on an RNA-binding compound that turned down PCSK9 production at the source. It’s like setting out to fix a leaky faucet and accidentally inventing a water purifier. Sometimes the cells know best!
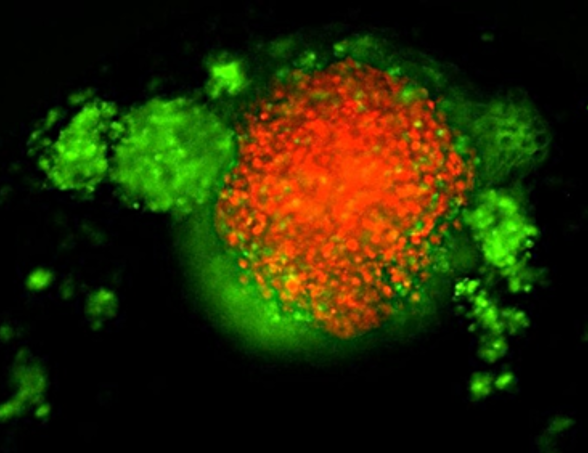
Pictured above: TNBC 3D spheroid expressing Kate2 (red) under attack by activated T cells (green).
Engineered cells talk back, and that’s a good thing!
These engineered systems aren’t just powerful, they’re versatile. Need to study a rare disease? You can tweak a reporter to mimic the condition. Want to screen for an entirely new drug class? Run a phenotypic assay and let the cells surprise you. Reporter cell assays are like the Swiss Army knife of drug discovery, only instead of a tiny scissors, you’ve got a glowing, signaling powerhouse. And if I’ve missed anything, such as reporters to track epigenetic changes or CRISPR-edited pathways, feel free to imagine it. The beauty of this field is that it’s a playground for creativity, where every new construct is a chance to push the boundaries of possibility.
So, why are engineered cells with reporter cell assays the unsung heroes of drug discovery? Because they’re fast, precise, and endlessly adaptable. They turn the chaos of cellular biology into a readable script, letting us spot the winners in a sea of compounds. They’ve given us numerous drugs, uncovered surprises like RNA binders, and keep the pipeline humming with potential. Sure, they might not get the Hollywood treatment but in the lab, they are real stars, shining a light on the next big breakthrough.
Isn’t that worth a round of applause? Or at least a celebratory glow from a luciferase assay?

Nicolas Ancellin is an experienced cell and molecular biologist specializing in drug discovery. He completed his post-doctoral fellowship at UCONN researching angiogenesis signaling before joining GlaxoSmithKline in 2002, where he successfully led multiple drug discovery projects and gained expertise in target identification and validation. Since joining Oncodesign in 2016, he has been instrumental in developing a LRRK2 kinase inhibitor, which is currently in Phase I clinical trials (run by OPM, sponsored by Servier). In his current role as the research site director and strategic project director at Oncodesign-services, Nicolas is actively involved in integrated drug discovery projects.