

In the dynamic landscape of oncology, innovative approaches are constantly emerging to tackle complex challenges. One such promising avenue gaining attention is the development of oncolytic viruses (OVs) which destroy cancer cells and leverage the host immune system to enhance the effect. The mechanism involves using modified viruses to stimulate an immune response. So let’s see what it is about and understand the good aspects of some viral infection.
Not all viruses are bad…
Because of their minimal simplistic structure and their parasitic nature, viruses are traditionally representing the frontier between living and non-living beings. They are classically categorized according to their genomic support (single or double stranded DNA or RNA, + or -), their shape and the presence of a lipid membrane. But at least from human perspective, they can be separated into three more simple categories: the good, the bad, and the not-so-ugly.
Viruses are mostly known as bad entities involved in infectious diseases by causing cell lysis resulting in tissue and organ destruction during their intense replication and proliferation.
For few of them, the “bad” ones, these infections are lethal without treatment (i.e. HIV, Ebola). More often, diseases are serious and rarely lethal in non-immunodeficient persons, like for SARS-COV2 or influenza virus. Alternatively, for viruses classified as oncogenic (HPV, EBV or HCV), an indirect adverse action may also occur: after integration into DNA and expression of viral oncogenes, oncovirus might also contribute the genesis of tumors. Another large group of viruses might be classified as neutral as their infection remains unnoticed or shows very light adverse symptoms (i.e. adenoviruses, responsible for cold, sore throat).

Good aspects of viral infections are less known. Nonetheless they are also essential for life
Since probably the beginning of life, viruses have essential roles in Darwinian diversity. Functional viruses enable horizontal exchanges of genomic material between species and even contributed to acquire essential characteristics in complex organisms (ie placenta, myelin). Remnant nonfunctional virus sequences representing a large part of non coding sequence of human genome are also very important for genome plasticity and evolution. Nowadays, defective viruses non-capable of replication are very often used as tools for GMO engineering, last step of evolution.
In the ecosystem, viruses have very important roles to regulate host population number. When the number is too high, viral infection and spreading is favored leading to reduction of population until it has decreased enough or has eventually been immunized. This is well described for algae bloom regulation in sea or river. It is also very important to regulate symbiotic relationship. For example, bacteriophages (bacterial viruses) regulates the adequate intestinal microbiota population growth essential for correct intestinal function and immune response. Bacteriophages are also getting more attention now as potential alternatives to antibiotics against multi-resistant bacterial pathogens.
The mechanisms behind oncolytic viruses
OVs belong to the category of not-so-ugly viruses. They are capable of targeting specifically tumor cells and eliminating them until their number is highly reduced or, more probably, until anti-viral immunity inactivates and eliminates them too.
As early as the beginning of the 20th century, cancer healing following viral infection was observed, but the first attempts to use wild type viruses as therapeutic approaches were not successful. Nowadays, the comprehension of molecular aspects of virus cycle mechanisms, cancer and immune system provides solutions to construct viruses enhanced to improve their oncolytic status, offering additional therapeutic tools against cancer.
Numerous viruses have already been tested in clinical studies as OVs. For historical reason, the most extensively used were initially adenoviruses (AV), Herpes simplex viruses (HSV) and vaccinia viruses (VV). This is why, T-VEC, the only worldwide approved oncolytic virus is HSV based. Four others OVs based on adenovirus or HSV are also now approved for clinical use but in single countries (China, Japan or USA). More recently, a dozen of other viruses were investigated pre-clinically and tested clinically like VSV, reoviruses, picornaviruses, most of them often chosen to show low virulence and to be absent in developed countries, to offer a sero-negative context in case of clinical use.
A very important characteristic allowing tumor infection of OVs over healthy tissues is that tumor cells are generally more sensitive to viruses. This increased vulnerability is due to the loss of their natural capacity to resist virus during acquisition of their tumoral status, by altering genes involved in apoptosis or immune cell recognition. For example, mutations in the IFN-JAK-STAT pathway favor tumor sensitivity.
Let’s go deeper in the mechanism…
Now, it is well admitted that the lytic activity of the OVs is not sufficient to eliminate the whole tumor but it is concomitant to anti-tumor immunization via innate and adaptative pathways to enable a full tumor regression. The activation is obtained by generating different changes in cascade: reversion of the exhausted/inactivated status of TILs (tumor infiltrated Lymphocytes) following cell lysis or due to anti-viral response, antigen presentation to dendrocytes, activation of macrophages, expression of cytokines, change in the TME (tumor microenvironment) at the levels of ECM (extracellular matrix) or CAF (cancer-associated fibroblasts)…
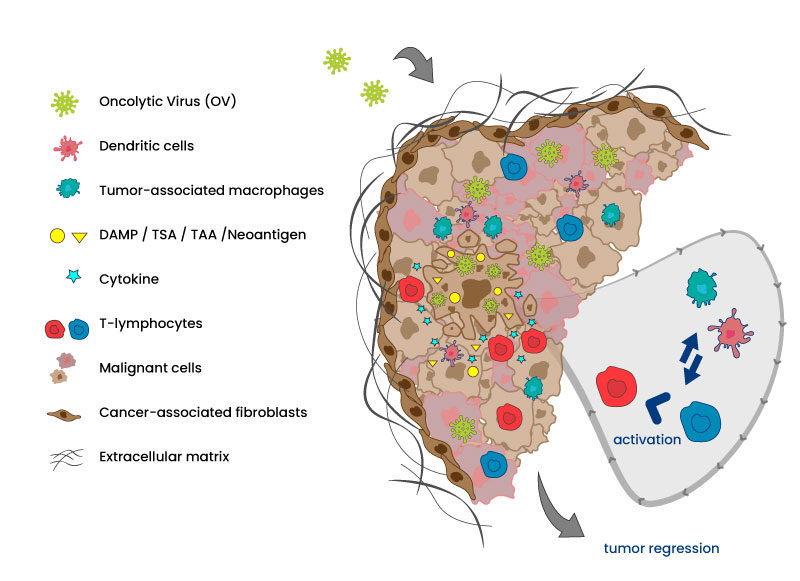
OVs specifically enter and lyse cancer cells. Lysis delivers chemoattractant molecules and tumor antigens that activates dendritic cells and stimulate chemokines secretion. Innate and adaptative immune system are awaken and recruited to also participate actively in tumor regression and eventually metastasis elimination.
Practically, wild-type versions of viruses are rarely used because they are often too dangerous or, when tolerated, their oncolytic potency is weak. The wild type viruses are now engineered by deleting or adding genes to optimize their oncolytic ability. The capacity of virus to integrate extra genome is an important feature to increase the number of exogenous genes. It can vary from very few genes to tens.
Removal of viral genes generally decreases their virulence, by suppressing their initial adverse tropism or reducing anti-viral immunity. OVs essentials promotors might also be engineered to be preferentially activated in tumor cells, favoring the right tropism for replication.
Gene insertions often shift OVs cell lysis from the non-immunogenic pathway preferred by WT virus towards ICD (Immunogenic Cell Death). Viruses can also express tumor associated antigens (TAA) or tumor specific antigens (TSA) or neoantigens to trigger a long-standing vaccination favorizing tumor shrinkage and preventing metastasis apparition or development. Second generation of OVs also express Bite (Bispecific T cell engagers) that attract T-cells to their tumoral targets or even ICI (Immune Checkpoint inhibitors) antibodies like anti-PD1 or anti-CTLA4 to reactivate existing T or NK cells inactivated in the TME.
Novel OVs are now frequently armed with pro-inflammatory cytokines. Two approved OVs express GM-CSF which promotes dendritic cell (DC) maturation. Numerous others cytokines, each with its own activation properties were also tested, from IL family to IFN, TNF and other chemokine molecules sometimes mutated or combined for optimal activation, with more or less success.
This number of possibilities results in the design of numerous OVs that have to be tested to find the right balance to:
- promote anti-cancer immunity to obtain full tumor regression but in the same time to decrease anti-viral immunity permitting reduction and multiplication of doses,
- remove any risk of lethal cytokine storm,
- maximize the immune stimulation in order to obtain an immune response that enable spatial (abscopal effect) and temporal (vaccination) responses to fight again metastasis and to prevent their reappearance,
- Enable a systemic injection preferred over tumoral injection for easy treatment of internal tumors and access to metastasis.
What about combination therapies?
Oncolytic viruses might be used in conjunction with other therapies to obtain synergistic combinations. They are often combined with ICI in order to further activates the adaptative response for vaccination. ICD might also be ameliorated by co-treatment with irradiation, some chemotherapies or targeted therapies against specific pathways. Targeted therapies may also increase the replication rate of viruses which increase their lytic potency and consequently the immune response. OVs have also been used successfully in combination with CAR-T cells, notably to promote their migration, infiltration and activation to potentially ameliorate their potency in solid tumors.
How to stimulate in preclinical studies?
All viruses are different. Each one has its own mechanism of action to escape immune system and to redirect cell synthesis and metabolism to selectively amplify its own genome and surround it by own capsid proteins. Similarly, each cancer is different and acquired specific set of mutations for immortalization and development of a pro-tumoral microenvironment, notably also in escaping immune response. As described above, numerous strategies exist to construct OVs efficient to eliminate tumors and their metastasis.
At Oncodesign Services, we have hundreds orthotopic and subcutaneous rodent or human CDX and PDX tumor models readily available to study the activity and mechanism of action of OVs in syngeneic models or humanized immune reconstituted models. Viruses can be manipulated in BSL2 and BSL3 status. Their proliferative properties might be followed by imaging or direct quantitation. Their immune response could be evaluated by flow cytometry or quantitation of cytokines. With 30 years of experience and thousands of studies performed, Oncodesign Services has also the know-how to provide support for testing combinations with other chemo, immuno-onco (ICI, ADC, BiTEs, CAR-T cells) or irradiation therapy to further increase the activity of OVs.
If you would like to know more about oncolytic viruses and how Oncodesign Services can help your projects, please get in touch using the contact form!
About the autor
This blog post was written by Dr Guillaume Serin, study director at Oncodesign Services. In his current role, Guillaume is involved in supporting oncolytic viruses’ program for customers.


