Why you should consider tumor microenvironment for cancer therapy?


June, 2023 l Olivier Duchamp & Sylvie Maubant
Development of drug resistance in cancer is related to several mechanisms of decreased drug efficacy, which are strongly influenced by intratumor heterogeneity and changes in the tumor microenvironment (TME). Tumor heterogeneity is one of the main factors behind therapeutic failures and fatal outcomes, and represents a major challenge to precision medicine goals. Although heterogeneity of cancer is conventionally attributed to genetic diversity by analogy with Darwinian evolution, current data reveal that, in addition to genetic factors, the tumor heterogeneity may derive from epigenetic alterations driven by stochastic events induced by physical and chemical signals from the TME.
Hereafter, we examine three essential players of the TME that are of increasing interest to the scientific community.
The TME is a highly structured ecosystem that surrounds cancer cells. It comprises diverse immune cells (e.g. T and B lymphocytes, macrophages, dendritic cells, natural killer cells, myeloid-derived suppressor cells, neutrophils and eosinophils), stromal cells (mainly fibroblasts), blood and lymphatic vessels, tissue-specific cells (e.g. neurons and adipocytes), microorganisms (with bacteria as most numerous members) along with the extracellular matrix and other secreted molecules (such as growth factors, cytokines, chemokines, metabolites). These components may vary by tissue type and co-evolve with the tumor development. Given the pivotal roles of the TME in tumor progression and regulating the efficacy of cancer treatment, strategies harnessing the TME have expanded significantly in recent years [1, 2].
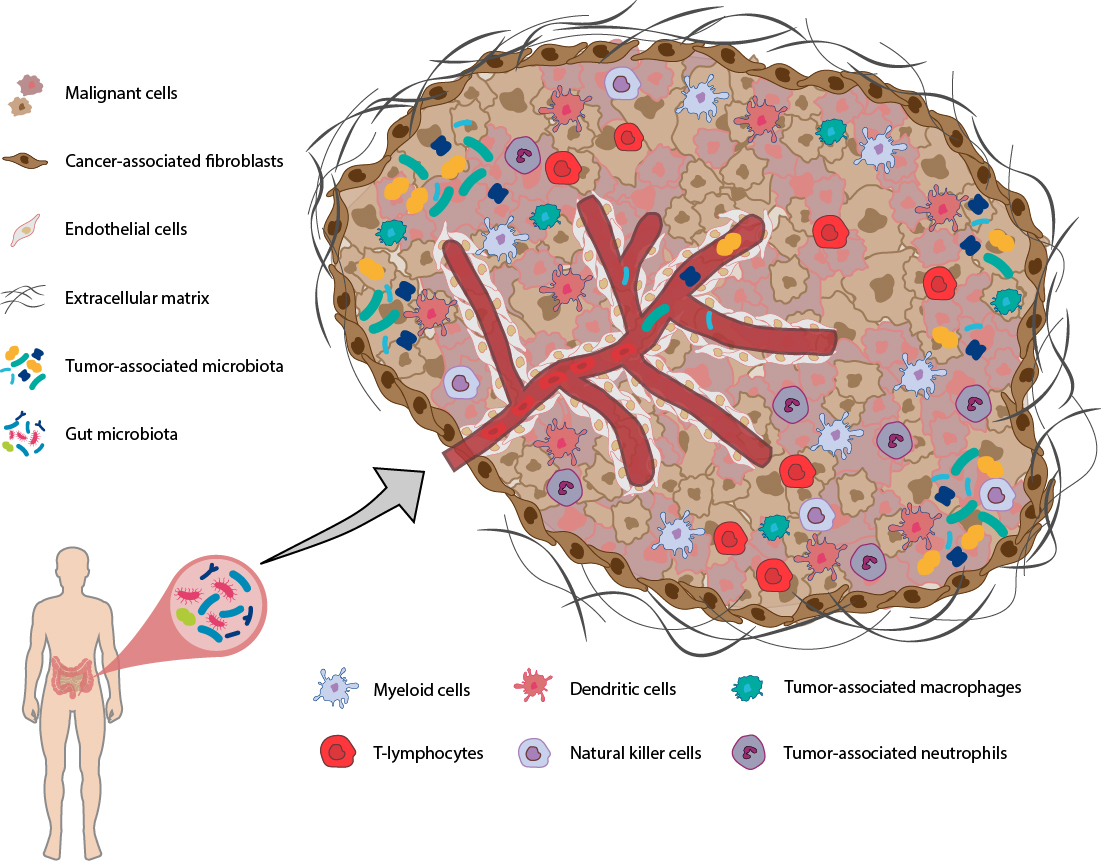
Given the pivotal roles of the TME in tumor progression and regulating the efficacy of cancer treatment, strategies harnessing the TME have expanded significantly in recent years
1. Tumor-associated macrophages (TAMs) |
 |
Tumor-associated macrophages (TAMs) constitute a major component of the leukocytic infiltrate in the TME. During tumor progression, TAMs are polarized from a M1-like to a M2-like phenotype in response to signals derived from TME. M2 TAMs actively inhibit proliferation and activity of cytotoxic T cells and promote expansion of T regulatory cells, thereby dampening the host immune responses against the tumor. In addition, these M2 TAMs have a key role in the orchestration of tumor proliferation and survival, angiogenesis and metastasis. Conversely, M1 TAMs can mediate phagocytosis of cancer cells and cytotoxic tumor killing.
Despite the spectrum of different functions that TAMs can execute, increased TAM abundance is most frequently associated with poor patient outcome and checkpoint inhibitor therapy resistance highlighting their potential as prognostic biomarkers and therapeutic targets. Several clinical trials combining checkpoint inhibitors and anti-CSF1R antibodies (expected inhibition of TAM proliferation and survival) or other TAM-based therapeutic strategies are ongoing [3, 4]. However, despite significant depletion of TAMs by anti-CSF1R treatment, differential anti-tumor effects can be observed and related to the TME phenotype.
For example, a screening on several syngeneic mouse tumor models has revealed that only the Renca murine kidney cancer model was responsive, a result explained by an increase of neutrophil infiltration within the tumor driven by chemokines which are secreted by fibroblasts following CSF1R inhibition [5, 6].
2. Cancer-associated fibroblasts (CAFs) |
 |
One of the most dominant components in the tumor stroma is CAFs, which not only provide physical support for tumor cells but also play a key role in promoting tumorigenesis or restraining cancer growth. CAFs can modulate recruitment and activity of innate and adaptive immune components within the TME along with extracellular matrix (ECM) deposition and remodeling.
Significant advances have been made in CAF-targeted therapies in recent years. These approaches mainly aim to (a) directly or indirectly deplete CAFs, (b) reduce or eliminate the tumor-promoting and immunosuppressive functions of CAFs, or (c) normalize or reprogram CAFs to a more quiescent state. Nevertheless, several challenges remain, such as identifying more specific CAFs markers, defining the functions and localization of the different CAF subpopulations during tumor progression, and finally developing agents specific enough to spare normal stromal cells in healthy tissues [7, 8].
To achieve this goal, still more predictive preclinical models need to be developed. At Oncodesign Services, we know that the stroma (i.e. mainly CAFs) is highly represented in rat tumors compared to mouse tumors and therefore likely rat models better mimic the clinical situation. However, in case of engraftment of a human tumor on those animals the human fibroblasts are rejected and replaced by rodent ones. It means that other technological alternatives such as organoid systems and 3D bioprinting which recapitulate the complex interactions in vitro between tumor and stroma have to also be considered for testing therapeutics targeting TME components [9].
3. Microbiota |
 |
Over the past decade, the number of studies demonstrating the important role of the intestinal microbiota in cancer development and response to therapy has grown significantly. Gut microbiota has been shown to regulate not only therapeutic response, but also the toxicity of several therapies, including chemotherapy, stem cell transplants and immunotherapy. For instance, several studies revealed that some gut microbiota signatures are associated with better response to immune checkpoint inhibitors, higher immune cell infiltration into tumors and enhanced systemic immunity [10, 11].
In recent years, the study of intratumoral microbiota has attracted more attention and made some progress, although more research is undeniably needed to better understand its role in cancer biology. Intratumoral microbiota has diverse sources (microorganisms originating from mucosal sites through mucosal barriers, from adjacent normal tissues or from organs including intestines and transported through the blood to tumor sites), organ composition and tissue distribution, and may be inextricably related to gut microbiota.
Accumulating evidence suggests that intratumoral microbiota plays a key role in shaping the local immune responses of the TME, which further affects tumor initiation and progression, immunotherapy efficacies and outcomes. The gut microbes act indirectly on the TME via their metabolites or the immune system, which may also impact the composition and function of the intratumoral microbiota thus adding here another level of crosstalk within the TME [12, 13].
Cancer results from an evolutionary adaptation of malignant cells to their microenvironment. Thus, a thorough understanding of the mechanisms mediating adaptive biological responses is crucial for progressing our war against cancer, in which neo-Darwinian and neo-Lamarckian models of adaptive evolution have to be considered to anticipate and to circumvent therapeutic resistance. And instead of killing tumor cells by debilitating drugs tailored to specific molecular targets, this approach should better support the development of therapies or novel drug combinations targeting multiple TME components aiming to prevent or alter the microenvironmental conditions that support cancer cells to survive.
This blog post was written by:
Do you want to exchange with our experts ?
[1] Bejarano et al (2021). Therapeutic targeting of the tumor microenvironment. Cancer Discov, 11: 933-59. DOI: 1158/2159-8290.cd-20-1808
[2] Tiwari et al (2022). Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J Biomed Sci, 29 (1): 83. DOI: 1186/s12929-022-00866-3
31] Mantovani et al (2022). Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov, 21 (11), 799-820. DOI: 1038/s41573-022-00520-5
[4] Mishra et al (2023). Macrophages as a potential immunotherapeutic target in solid cancers. Vaccines, 11 (1): 55. DOI: 3390/vaccines11010055
[5] Kumar et al (2017). Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell, 32 (5): 654-68. DOI: 1016/j.ccell.2017.10.005
[6] O’Brien et al (2021). Activity of tumor‑associated macrophage depletion by CSF1R blockade is highly dependent on the tumor model and timing of treatment. Cancer Immunol Immunother, 70: 2401-10. DOI: 1007/s00262-021-02861-3
[7] Sahai et al (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer, 20 (3): 174-86. DOI: 1038/s41568-019-0238-1
[8] Glabman et a (2022). Cancer-associated fibroblasts: tumorigenicity and targeting for cancer therapy. Cancers, 14 (16): 3906. DOI: 3390/cancers14163906
[9] Rodrigues et al (2021). 3D in vitro model (r)evolution: unveiling tumor-stroma interaction. Trends Cancer, 7 (3): 249-64. DOI: 1016/j.trecan.2020.10.009
[9] Routy et al (2018). The gut microbiota influences anticancer immunosurveillance and general health. Nat Rec Clin Oncol, 15 (6): 382-96. DOI: 1038/s41571-018-0006-2
[10] Fernandes et al (2022). Targeting the gut microbiota for cancer therapy. Nat Rev Cancer, 22: 703-22. DOI: 1038/s41568-022-00513-x
[11] Sepich-Poore et al (2021). The microbiome and human cancer. 371 (6536): eabc4552. DOI: 10.1126/science.abc4552
[12] Yang et al (2023). Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther, 8 (1): 35. DOI: 1038/s41392-022-01304-4
oncology, tumor microenvironment, tumor-associated macrophages, cancer-associated fibroblasts, tumor microbiota, gut microbiota