Ask the Expert:

A Q&A with Caroline Mignard, Study Director in Translational Pharmacology
As oncology drug development becomes more precise and patient-focused, humanized models have emerged as a game-changing tool in preclinical research. These advanced in vivo models enable researchers to simulate human immune responses and tumor behavior with more accuracy, – enabling more predictive, translatable results.
At Oncodesign Services, we support biopharma innovators in accelerating oncology breakthroughs. Humanized models are a cornerstone of that mission. We exchanged with Caroline Mignard, Study Director in Translational Pharmacology, to explore what you should know when working with these models in oncology.
Q: Caroline, What exactly are humanized models?
Caroline: In simple terms, a humanized model contains something human, whether it’s human cells, human tumors, or both. 
Historically, traditional models, like syngeneic mouse models, weren’t sufficient for answering all scientific questions, especially in immuno-oncology. So, researchers began using human tumor grafts. But this introduced a challenge: the mouse immune system would reject human cells.
To overcome this, model providers developed genetically modified, immunodeficient mice, allowing us to introduce human immune cells or tissues without rejection. These models now allow us to simulate human-like immune responses, critical for evaluating immunotherapies and combination strategies.
Humanized models help bridge the translational gap by enabling a more accurate and relevant evaluation of oncology drugs, especially in immune-oncology.
Q: What is the main challenge for biopharma innovators?
Caroline: The biggest challenge is having the right model, one that is both relevant to the therapeutic strategy and predictive of human clinical outcomes.
There’s no one-size-fits-all approach. The ideal model depends on the mechanism of action, tumor type, and study objectives. Sometimes, even a negative result can be just as valuable as a positive one, as it helps de-prioritize a candidate and avoid unnecessary clinical investment.
Model selection is critical. It must be tailored to the biological question, or you risk generating misleading or inconclusive data.
Q: What do you typically evaluate with humanized models?
Caroline: Humanized models are particularly valuable in immuno-oncology, where understanding how the human immune system interacts with tumor cells is vital. These models allow researchers to study:
- T-cell activation and exhaustion
- Cytokine signaling
- Immune checkpoint modulation
- Tumor growth and immune evasion
Importantly, they also allow for exploration of the tumor microenvironment (TME), a major factor in drug resistance and therapeutic failure. With humanized models, we can assess the complex interactions between cancer cells, immune cells, and stromal components in vivo.
The predictive power of humanized models helps reduce attrition rates, improve patient selection strategies, and de-risk pipeline assets before clinical trials.
Q: What’s next for humanized models in oncology?
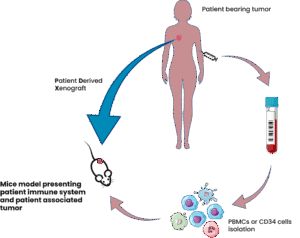
Caroline: The field is moving fast, and one of the most promising frontiers is the development of autologous models, where both the tumor and immune cells are derived from the same human donor. While technically complex, this approach could significantly reduce donor variability and better reflect individual patient biology. It’s a step toward more personalized and precise preclinical evaluation.
In parallel, there’s growing interest in alternative models such as organoids and ex vivo systems. These tools complement in vivo research but, for now, humanized mouse models remain essential for studying the full complexity of immune-tumor interactions in a living system.
Our biggest challenge is keeping pace with innovation by —continuously evaluating new technologies and refining our models to stay ahead of therapeutic needs.
Q: What about regulatory and ethical considerations?
Caroline: Humanized animal models are living beings entrusted to us for advancing scientific discovery, and their welfare is our highest ethical responsibility. These animals are more physiologically delicate than standard models and require attentive care, enriched environments, and close, expert monitoring. At Oncodesign Services, our commitment goes beyond regulatory obligations—we work to ensure each model receives humane, compassionate treatment throughout the study process. All our sites are AAALAC accredited, and our protocols are fully aligned with European regulations. We uphold the 3Rs principles as a foundation of our work:
- Replacement: seeking scientifically sound alternatives whenever possible
- Reduction: designing studies that minimize animal use without compromising data quality
- Refinement: continuously improving procedures to reduce stress and promote well-being
Ethics are at the core of our work. It’s not just about regulatory compliance—it’s about doing science responsibly.
Ready to take your oncology program to the next level
At Oncodesign Services, we combine decades of oncology expertise with cutting-edge humanized model platforms. Whether you’re advancing a novel immunotherapy or exploring resistance mechanisms, our customized preclinical solutions generate high-impact data, clinically relevant data to guide your success. To learn more about our range of humanized models, you can download a technical sheet on what to expect from humanized models
Caroline Mignard is a pharmacologist in preclinical oncology research at Oncodesign Services. She has obtained a Ph.D in Biology from the University of Strasbourg in France. She was involved in a French collaborative project (IMODI) to establish new animal models such as PDX and humanized mice for cancer research. Her role now at Oncodesign Services is to manage a team of Study Directors and to provide scientific expertise to researchers for the evaluation of their candidate through preclinical studies. She is the co-author of several publications about PDX models.