Preclinical CRO Services for Inflammatory Lung Diseases
Acute and chronic inflammatory lung diseases are among the most common medical conditions in the world. Rodent models of lung inflammation are helpful to decipher the mechanisms underlying the triggering and/or sustaining of the disease observed in patients.
Oncodesign Services offers several preclinical models available as CRO services, addressing a variety of lung diseases, and provides CRO support for the de novo development of new lung inflammation models recently described in literature.
Typical readouts for lung inflammation
- Body weight
- Lung weight
- Lung weight-to-body weight ratio
- Clinical scoring
- Edema
- Fibrosis score
- Mucus production
- Viral load
- Ex vivo lung function, including compliance and elasticity (FlexiVent, SCIREQ)
- Broncho-alveolar fluid (BALF) cell count and cytokine secretion
- CT scan
- Gene expression in lungs, by qPCR/dPCR
- Biomarker / drug monitoring
Common drug administration routes (PO, IV, IP) and also non-typical drug administration routes used for lung therapies:
- Intra-tracheal
- Intra-nasal
- Osmotic mini-pumps
Discover Oncodesign Services offers for lung inflammation diseases
-
In Vitro capabilities
- Cells:
- HPASMC (Human Pulmonary Arterial Smooth Muscle Cells)
- HPAEC (Human Pulmonary Arterial Endothelial Cells)
- Assays:
- Collagen formation (scar-in-a-jar assay)
- Impedance (signaling) assay
- Epithelial–mesenchymal Transition assay
- Surrogate of pulmonary arterial hypertension (PAH)
- Cells:
-
In Vivo Models
Oncodesign Services has developed robust models to provide CRO services that deliver high quality data.
- Bronchiolitis model (1) in mice, induced by Human Respiratory Syncytial Virus (RSV)
- Allergic asthma model (2) in mice, induced by House Dust Mice (HDM)
- Corticoid-resistant asthma (3) in mice, induced by Ovalbumin (OVA) + Influenzae
- Asthma exacerbation model (4) in mice, induced by RSV + HDM combination
- Lung fibrosis model (5) in mice, induced by Bleomycin delivered IT or by osmotic pumps
If you cannot find the model you desire in this list, please get in touch.
Case studies for lung inflammation
-
#1 House dust-mite (HDM)-induced allergic asthma model
HDM is one of the most important sources of indoor allergens and a significant factor underlying allergic rhinitis and allergic asthma. HDM intranasal challenge can be used in BALB/c mice to reproduce many key features of clinical asthma:
- HDM challenge (IN) administered 5 days per week during 3 weeks
- Elevated levels of IgE
- Airway inflammation
- Goblet cell hyperplasia with mucus overproduction
- Release of inflammatory mediators and cytokines primarily associated with Th2-type inflammation
- Responsive to fluticasone (inhaled corticoid)
Results show allergic signature in bronchoalveolar lavage fluid (BALF) and in plasma with BALF cell content & cytokine secretion, and with plasma IgE levels.
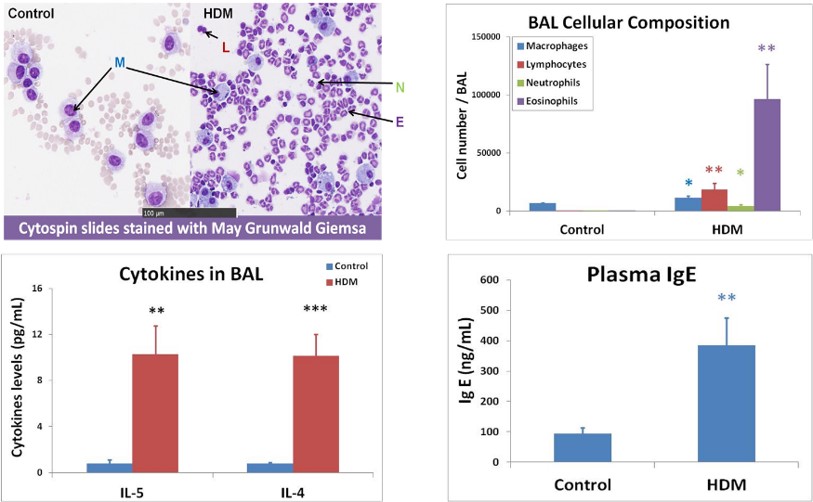
Allergic signature inbronchoalveolar lavage (BAL) and in plasma :
- BAL cell content & cytokine secretion
- Plasma IgE levels
-
#2 Bronchiolitis model – Respiratory syncytial virus (RSV) infection in BALB/c mice
Respiratory syncytial virus (RSV) is a virus responsible for severe respiratory symptoms including rhinitis, bronchiolitis and pneumonia. About 65% of children are infected with RSV within the first year of life.
The disease is modeled by delivering a high dose of RSV by intranasal instillation to BALB/c mice, which allows viral infection and bronchiolitis symptoms. Excessive production of airway mucus is a feature of such inflammatory lung disease and is a key histology readout. Ribavirin is typically used as a reference compound.
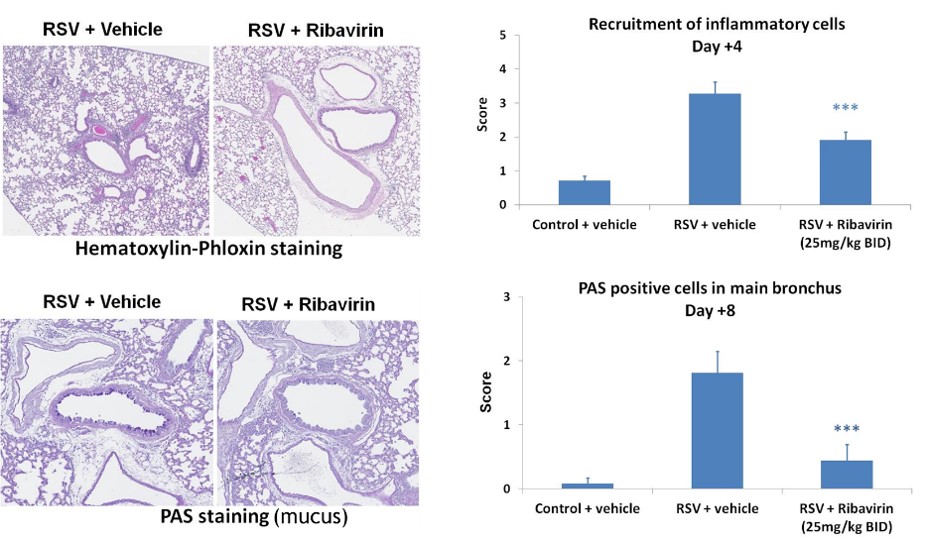
-
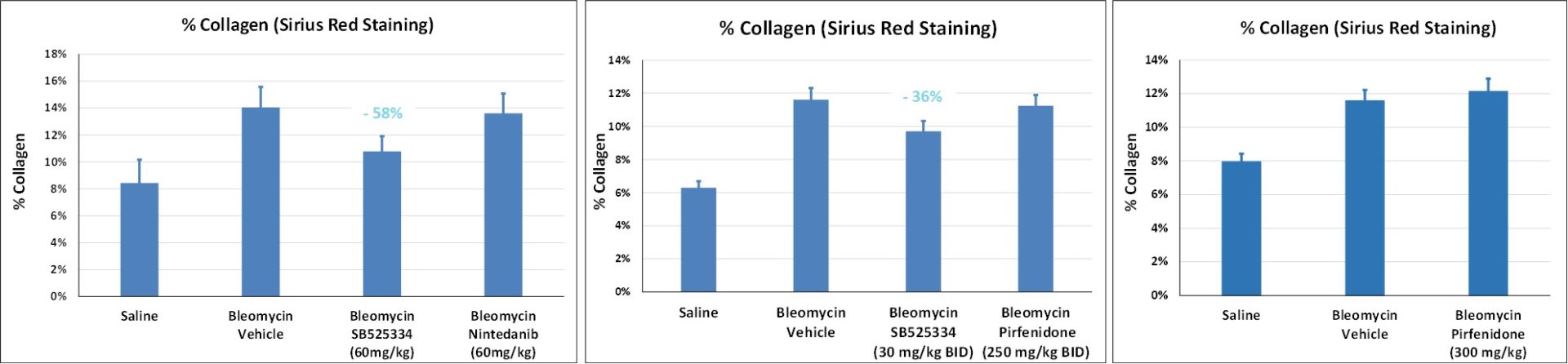
#3 Bleomycin-induced lung fibrosis model using intra-tracheal instillation
To date, the mouse bleomycin-induced lung injury model remains the most frequently used animal model to investigate pulmonary fibrosis resulting from inflammation.
Similar to the human disease, bleomycin exposure in mice is is associated to epithelial damage, inflammatory cell infiltration, and proliferation of fibroblasts and myofibroblasts, as well as extra-cellular matrix excessive deposition.
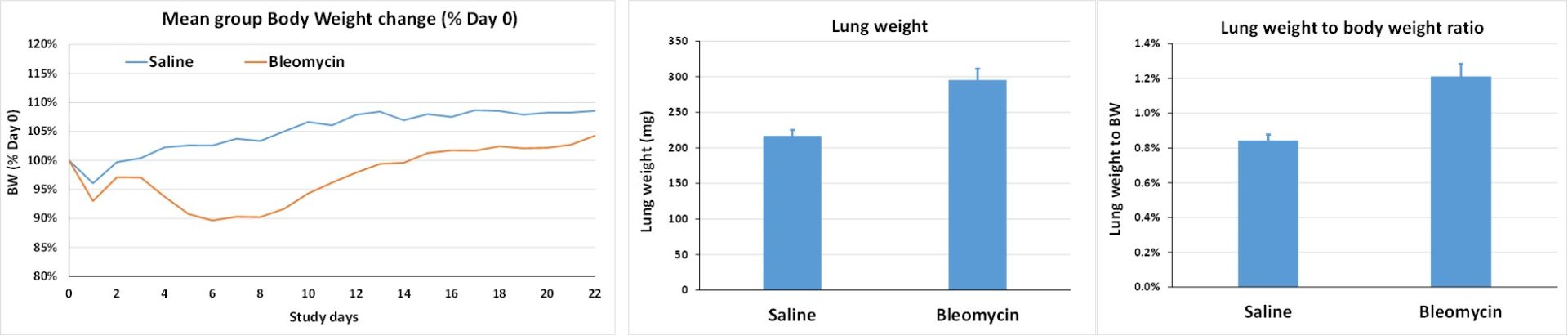
Model setup: intra-tracheal (IT) administration of bleomycin on Day 0 in C57BL/6 male mice (n=8 animals/group as a minimum after randomization).
CRO study design to obtain robust data
- D0: single IT administration of bleomycin or saline under ketamine/xylazine anesthesia
- D7: selection of good responders to bleomycin (based on % body weight loss)
- Selection rate of ±65% – around 12 animals per group to get 8 selected animals per group
- D7 to D21: treatment
- Around D21: termination of animals
Lung histology at D21 for anti-fibrotic drug evaluation
- ALK5 (TGFb receptor I) inhibitor [SB-525334]: effective at 30 or 60mg/kg/day (with Cmax-driven dose-effect)
- Nintedanib (Tyr kinase inhibitor): no effect at 60 or 100mg/kg/day
- Pirfenidone (molecular target unknown): no effect at 300 or 500mg/kg/day
-
References
(1) Bronchiolitis model in mice, induced by Human Respiratory Syncytial Virus (RSV)
Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013 Dec;45(3):331-79. doi: 10.1007/s12016-013-8368-9. PMID: 23575961; PMCID: PMC7090643.
https://pubmed.ncbi.nlm.nih.gov/23575961/
https://link.springer.com/article/10.1007/s12016-013-8368-9
Taylor G. Animal models of respiratory syncytial virus infection. Vaccine. 2017 Jan 11;35(3):469-480. doi: 10.1016/j.vaccine.2016.11.054. Epub 2016 Nov 29. PMID: 27908639; PMCID: PMC5244256.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5244256/
Ceneviva ZJ, Norlander AE, Stokes Peebles R Jr. Mouse Models of Respiratory Syncytial Virus Infection. Methods Mol Biol. 2022;2506:19-41. doi: 10.1007/978-1-0716-2364-0_2. PMID: 35771461.
https://pubmed.ncbi.nlm.nih.gov/35771461/
Tregoning JS, Yamaguchi Y, Harker J, Wang B, Openshaw PJ. The role of T cells in the enhancement of respiratory syncytial virus infection severity during adult reinfection of neonatally sensitized mice. J Virol. 2008 Apr;82(8):4115-24. doi: 10.1128/JVI.02313-07. Epub 2008 Feb 13. PMID: 18272579; PMCID: PMC2293007.
https://pubmed.ncbi.nlm.nih.gov/18272579/
Sebina I, Phipps S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses. 2020 Jul 27;12(8):808. doi: 10.3390/v12080808. PMID: 32726921; PMCID: PMC7472258.
https://pubmed.ncbi.nlm.nih.gov/32726921/
(2) Allergic asthma model in mice, induced by House Dust Mice (HDM)
Gao X, Leung TF, Wong GW, Ko WH, Cai M, He EJ, Chu IM, Tsang MS, Chan BC, Ling J, Fan X, Lu L, Lam CW, Wong CK. Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma. Cell Mol Immunol. 2022 Feb;19(2):245-259. doi: 10.1038/s41423-021-00803-8. Epub 2021 Nov 30. PMID: 34848868; PMCID: PMC8803866.
https://pubmed.ncbi.nlm.nih.gov/34848868/
Debeuf N, Haspeslagh E, van Helden M, Hammad H, Lambrecht BN. Mouse Models of Asthma. Curr Protoc Mouse Biol. 2016 Jun 1;6(2):169-184. doi: 10.1002/cpmo.4. PMID: 27248433.
https://pubmed.ncbi.nlm.nih.gov/27248433/
Oikonomou N, Schuijs MJ, Chatzigiagkos A, Androulidaki A, Aidinis V, Hammad H, Lambrecht BN, Pasparakis M. Airway epithelial cell necroptosis contributes to asthma exacerbation in a mouse model of house dust mite-induced allergic inflammation. Mucosal Immunol. 2021 Sep;14(5):1160-1171. doi: 10.1038/s41385-021-00415-5. Epub 2021 May 27. PMID: 34045680; PMCID: PMC8379077.
https://pubmed.ncbi.nlm.nih.gov/34045680/
Ma M, Li G, Qi M, Jiang W, Zhou R. Inhibition of the Inflammasome Activity of NLRP3 Attenuates HDM-Induced Allergic Asthma. Front Immunol. 2021 Aug 3;12:718779. doi: 10.3389/fimmu.2021.718779. PMID: 34413860; PMCID: PMC8369415.
https://pubmed.ncbi.nlm.nih.gov/34413860/
Maes B, Smole U, Vanderkerken M, Deswarte K, Van Moorleghem J, Vergote K, Vanheerswynghels M, De Wolf C, De Prijck S, Debeuf N, Pavie B, Toussaint W, Janssens S, Savvides S, Lambrecht BN, Hammad H. The STE20 kinase TAOK3 controls the development of house dust mite-induced asthma in mice. J Allergy Clin Immunol. 2022 Apr;149(4):1413-1427.e2. doi: 10.1016/j.jaci.2021.08.020. Epub 2021 Sep 8. PMID: 34506849.
https://pubmed.ncbi.nlm.nih.gov/34506849/
(3) Corticoid-resistant asthma in mice, induced by Ovalbumin (OVA) + Influenzae
Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, Foster PS, Hansbro PM. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012 Jul;67(7):588-99. doi: 10.1136/thoraxjnl-2011-200160. Epub 2012 Mar 3. PMID: 22387445.
https://pubmed.ncbi.nlm.nih.gov/22387445/
Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR, Hansbro NG, Hirota JA, Wood LG, Simpson JL, Knight DA, Wark PA, Gibson PG, O’Neill LAJ, Cooper MA, Horvat JC, Hansbro PM. Role for NLRP3 Inflammasome-mediated, IL-1β-Dependent Responses in Severe, Steroid-Resistant Asthma. Am J Respir Crit Care Med. 2017 Aug 1;196(3):283-297. doi: 10.1164/rccm.201609-1830OC. PMID: 28252317.
https://pubmed.ncbi.nlm.nih.gov/28252317/
(4) Asthma exacerbation model in mice, induced by RSV + HDM combination
Zhang D, Yang J, Zhao Y, Shan J, Wang L, Yang G, He S, Li E. RSV Infection in Neonatal Mice Induces Pulmonary Eosinophilia Responsible for Asthmatic Reaction. Front Immunol. 2022 Feb 2;13:817113. doi: 10.3389/fimmu.2022.817113. PMID: 35185908; PMCID: PMC8847141.
https://pubmed.ncbi.nlm.nih.gov/35185908/
https://www.frontiersin.org/articles/10.3389/fimmu.2022.817113/full
Makino A, Shibata T, Nagayasu M, Hosoya I, Nishimura T, Nakano C, Nagata K, Ito T, Takahashi Y, Nakamura S. RSV infection-elicited high MMP-12-producing macrophages exacerbate allergic airway inflammation with neutrophil infiltration. iScience. 2021 Oct 2;24(10):103201. doi: 10.1016/j.isci.2021.103201. PMID: 34703996; PMCID: PMC8524145.
https://pubmed.ncbi.nlm.nih.gov/34703996/
Matsuse H, Hirose H, Tsuchida T, Fukahori S, Fukushima C, Mizuta Y, Kohno S. Effects of respiratory syncytial virus infection on dendritic cells and cysteinyl leukotrienes in lung tissues of a murine model of asthma. Allergol Int. 2007 Jun;56(2):165-9. doi: 10.2332/allergolint.O-06-476. Epub 2007 May 1. PMID: 17460444.
https://pubmed.ncbi.nlm.nih.gov/17460444/
(5) Lung fibrosis model in mice, induced by Bleomycin delivered IT or by osmotic pumps
Herrmann FE, Hesslinger C, Wollin L, Nickolaus P. BI 1015550 is a PDE4B Inhibitor and a Clinical Drug Candidate for the Oral Treatment of Idiopathic Pulmonary Fibrosis. Front Pharmacol. 2022 Apr 20;13:838449. doi: 10.3389/fphar.2022.838449. PMID: 35517783; PMCID: PMC9065678.
https://www.frontiersin.org/articles/10.3389/fphar.2022.838449/full
Lee R, Reese C, Bonner M, Tourkina E, Hajdu Z, Riemer EC, Silver RM, Visconti RP, Hoffman S. Bleomycin delivery by osmotic minipump: similarity to human scleroderma interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2014 Apr 15;306(8):L736-48. doi: 10.1152/ajplung.00210.2013. Epub 2014 Feb 28. PMID: 24583879; PMCID: PMC3989726.
https://pubmed.ncbi.nlm.nih.gov/24583879/
Liang M, Lv J, Zou L, Yang W, Xiong Y, Chen X, Guan M, He R, Zou H. A modified murine model of systemic sclerosis: bleomycin given by pump infusion induced skin and pulmonary inflammation and fibrosis. Lab Invest. 2015 Mar;95(3):342-50. doi: 10.1038/labinvest.2014.145. Epub 2014 Dec 15. PMID: 25502178.
https://www.nature.com/articles/labinvest2014145
Hübner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques. 2008 Apr;44(4):507-11, 514-7. doi: 10.2144/000112729. PMID: 18476815.
https://pubmed.ncbi.nlm.nih.gov/18476815/
Egger C, Gérard C, Vidotto N, Accart N, Cannet C, Dunbar A, Tigani B, Piaia A, Jarai G, Jarman E, Schmid HA, Beckmann N. Lung volume quantified by MRI reflects extracellular-matrix deposition and altered pulmonary function in bleomycin models of fibrosis: effects of SOM230. Am J Physiol Lung Cell Mol Physiol. 2014 Jun 15;306(12):L1064-77. doi: 10.1152/ajplung.00027.2014. Epub 2014 Apr 11. PMID: 24727584.
https://pubmed.ncbi.nlm.nih.gov/24727584/
De Langhe E, Cailotto F, De Vooght V, Aznar-Lopez C, Vanoirbeek JA, Luyten FP, Lories RJ. Enhanced endogenous bone morphogenetic protein signaling protects against bleomycin induced pulmonary fibrosis. Respir Res. 2015 Mar 15;16(1):38. doi: 10.1186/s12931-015-0202-x. PMID: 25849157; PMCID: PMC4364322.
https://pubmed.ncbi.nlm.nih.gov/25849157/
Dackor RT, Cheng J, Voltz JW, Card JW, Ferguson CD, Garrett RC, Bradbury JA, DeGraff LM, Lih FB, Tomer KB, Flake GP, Travlos GS, Ramsey RW Jr, Edin ML, Morgan DL, Zeldin DC. Prostaglandin E₂ protects murine lungs from bleomycin-induced pulmonary fibrosis and lung dysfunction. Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L645-55. doi: 10.1152/ajplung.00176.2011. Epub 2011 Aug 19. PMID: 21856819; PMCID: PMC3213994.
https://pubmed.ncbi.nlm.nih.gov/21856819/
Phillips JE, Peng R, Burns L, Harris P, Garrido R, Tyagi G, Fine JS, Stevenson CS. Bleomycin induced lung fibrosis increases work of breathing in the mouse. Pulm Pharmacol Ther. 2012 Aug;25(4):281-5. doi: 10.1016/j.pupt.2011.10.001. Epub 2011 Oct 20. Erratum in: Pulm Pharmacol Ther. 2022 Jun;73-74:102122. PMID: 22024054.
https://pubmed.ncbi.nlm.nih.gov/22024054/