Integrated CRO Solutions for Immuno-Oncology Programs
Oncodesign Services offers a full suite of in vitro, in vivo and bioanalytical capabilities to serve the integrated needs of immuno-oncology (IO) programs. We have served the special needs of bispecific, CAR-T and other Immunno-Oncology therapies since 2002.
Special Needs of Immuno-oncology Programs
Therapies aiming to modulate immune cells within the tumor microenvironment require host animals with functional immune compartments. The most popular in vivo approaches to modeling tumor-immune interactions are with syngeneic mouse tumor models and humanized mice bearing CDX or PDX. Each of these approaches has advantages and constraints, with Oncodesign Services tailoring a development strategy to best fit the needs of the Immuno-Oncology (IO) program.
Syngeneic studies are easy to perform, but may not be suitable if the therapy does not have mouse cross-reactivity. Syngeneic models are popular, and due to the long experience at Oncodesign Service, we have characterized these models thoroughly for drug responses. Browse our list of syngeneic models and their responses to SOC therapies on the Tumor Bank page.
Syngeneic murine tumor models are efficient drug develpment tools, comprising of murine components (tumor antigens, murine effectors cells, etc) and enable the murine immune system and murine tumors to interact within a fully functional tumor microenvironment (TME). The limitations of use include situations when 1) therapeutic strategies target the human compartment only and 2) drugs are fully-humanized (antibodies, bispecifics, cellular therapies, gene therapies).
Because humanization of the entire immune compartment is incomplete, a mouse experiment needs to consider the appropriate human immune reconstitution to investigate MOA of drugs in specific tumor microenvironment (TME). Humanized models have more variability and offer only a narrow window of therapeutic response before Graft-versus-Host-Disease (GvHD) or anemia occurs (typically 4-6 weeks after engraftment for PBMC-humanized models or 8 weeks after humanization onset for HSC-humanized models, respectively).
Typical Analytical Methods & Readouts in Immuno-Oncology programs
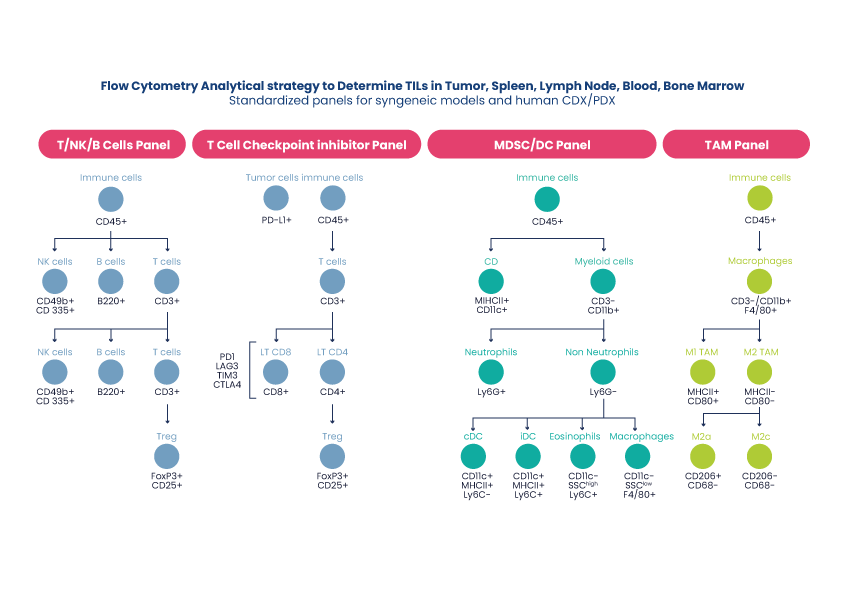
Like all oncology studies, the most important readouts in immuno-oncology are tumor size and survival. However, immuno-oncology studies typically bundle ex vivo analysis of the tumor-infiltrating lymphocytes (TILs) and cytokine profiling of blood serum. Fresh, dissociated tumors, lymph nodes and terminal blood are analyzed by flow/FACS and FFPE samples by IHC to understand the stroma and tumor microenvironment (TME).
Read about Oncodesign Service’s biomarker platform here.
In vitro Immuno-Oncology Assays
Recapitulation of the TME in vitro remains a lofty goal still to be validated translationally. However, some more modest in vitro approaches short of stromal reconstitution might still be useful to de-risk Immuno-oncology therapies before in vivo studies:
- Phenotypic response of tumor cells alone or PBMCs alone, to a titrated treatment, in vitro, with readouts by cell imaging, flow/FACS or well-based readout. E.g. Antibody-mediated apoptosis. Gene expression readouts are also popular.
- Mechanistic co-cultures of tumor cells and select immune populations, for purposes of MoA studies. E.g. Dendritic cell and tumor cell co-cultures for phagocytosis study. Also validated for NK-cell and tumor cell co-cultures.
- A step-wise panel of known inducers and response mechanisms, validated for a limited range of known-responsive CDX. E.g. Immunogenic cell death assays.
Example #1:
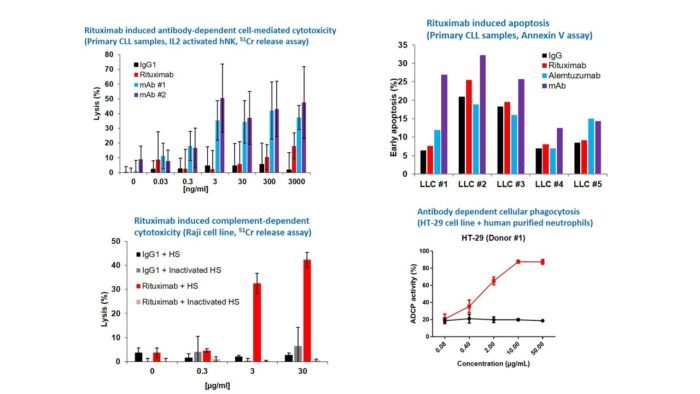
A range of tumor cell models were treated with antibody Rituximab, which demonstrated a range of different tumor killing mechanisms. Rituximab induced apoptosis in primary CLL tumor samples. Rituximab induced complement dependent cytotoxicity (CDC) in Raji tumor cell line. Another antibody test article induced antibody dependent cellular phagocytosis (ADCP) in HT-29 and human purified neurophils. Antibody dependent cell cytotoxicity (ADCC) assays also available. These assays can be adapted for cell therapies, e.g. NKs for ADCC, macrophages or neutrophils for ADCP.
Example #2:
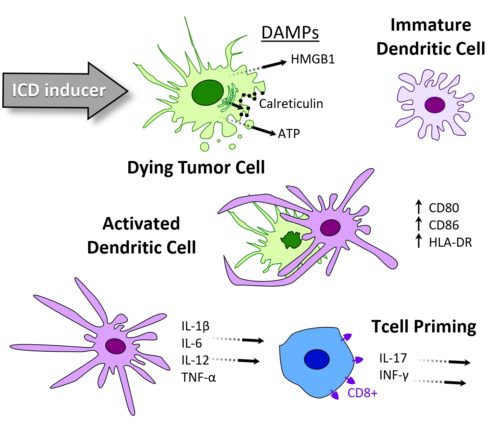
Immunogenic cell death (ICD) is a type of tumor death resulting in persistent immune response, and is thus an endogenous mechanism of cancer vaccination. Oncodesign Services’ ICD platform is a three step assay cascade, developed to characterize compounds for their ICD inducer potential. Step #1 involves the characerization of tumor cell death, step #2 confirms dentritic cell activition and step #3 quantifies T cell priming. Not all CDX respond to ICD inducers. Oncodesign Services first developed this platform in 2017 and has now identified several mouse and human CDX able to be used as models for ICD. Please inquire.
Example #3:
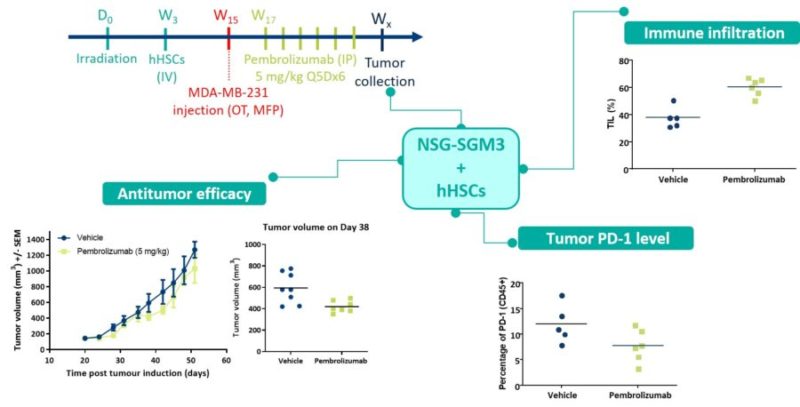
An immune checkpoint inhibitor Pembroizumab was tested for efficacy using the SC breast MDA-MB-231 model in hHSC humanized NSG-SGM3 mice. From the tumor size readout alone, the drug appeared to make a slight improvement to the tumor growth, but the immune infiltration data show that about 60% of the treated tumor volume consisted of immune cells. These results indicated the drug was more effective than it first appeared, with tumor shrinkage masked by immune infiltrate volume. Quantification of PD-1 in the stromal tissue confirmed that the checkpoint inhibitor worked to suppress checkpoint expression.
Example #4:
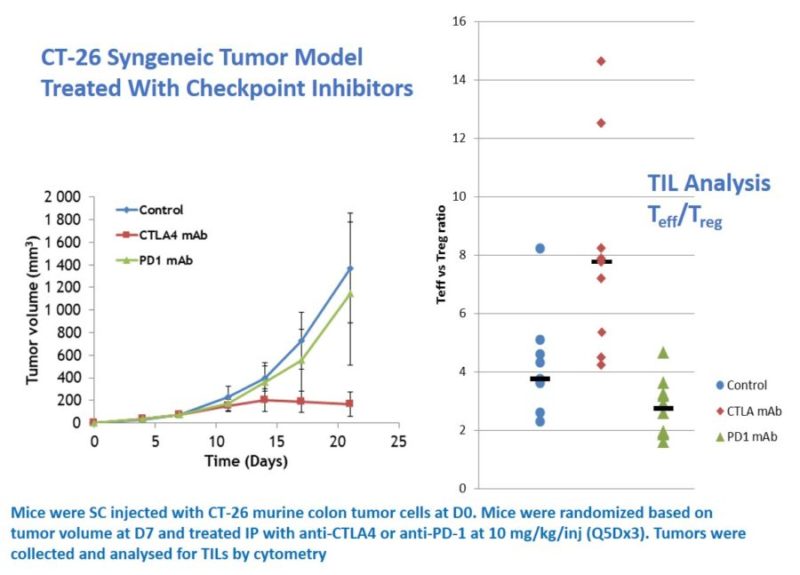
Mice were injected with CT-26 murine colon tumor cells on D0. Mice were randomized based on tumor volume at D7 and treated IP with anti-CTLA4 antibody or anti-PD-1 to study their anti-tumor activity. Tumor volumes were measured daily, with tumors dissociated for TIL analysis by flow/FACS. These analyses showed that the anti-CTLA4 antibody significantly changed the Teff/Treg ration in the TME.