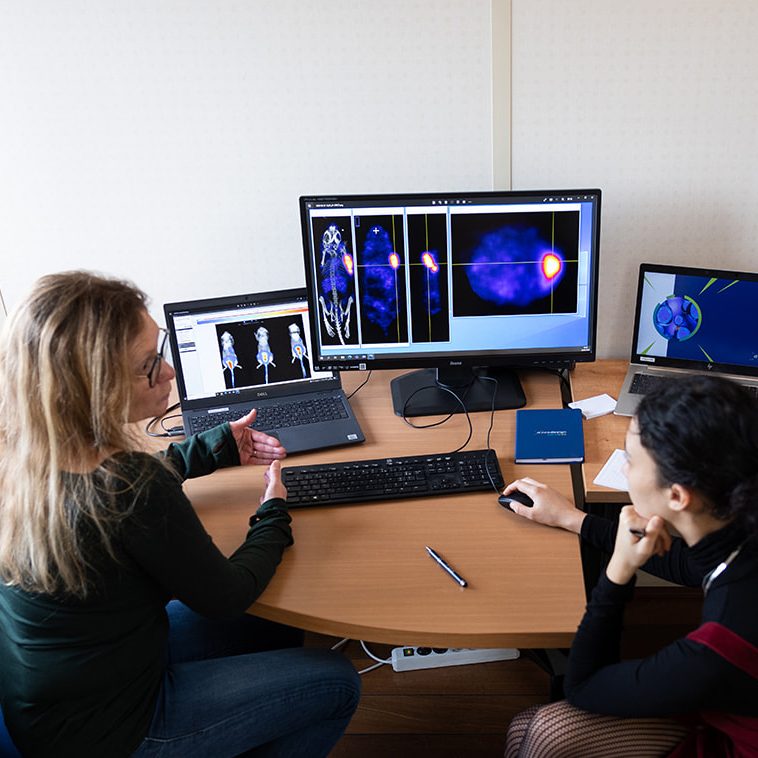


Targeted Radiotherapy Topics That Stood Out in Barcelona
The 2025 EANM Annual Congress in Barcelona once again confirmed how fast the radiopharmaceutical landscape is evolving. From the growing enthusiasm around terbium-161 and astatine-211 to renewed interest in combination therapies and pretargeting strategies, this year’s discussions reflected a maturing field, one that is ready to scale but still facing practical challenges.
Here’s what caught our attention.
Terbium Moves Into the Spotlight
If one radionuclide stole the show this year, it was terbium-161. Across multiple sessions, researchers compared its performance with the long-established lutetium-177, and the consensus was clear: terbium is showing superior tumor-killing efficacy in preclinical and early clinical studies.
This echoes our own findings, where terbium has reliably outperformed lutetium in efficacy studies. The isotope’s dual emission profile, combining beta particles and short-range Auger electrons, gives it a stronger local therapeutic impact on tumor cells while sparing healthy tissue. An additional advantage of terbium lies in its simultaneous ability for SPECT imaging, demonstrating its theranostic versatility.
The challenge, however, remains in its accessibility. Lutetium is still easier to source and handle, while terbium production is limited to a handful of sites in Europe. But with mounting evidence and enthusiasm, terbium is poised to become a major player as the supply chains expand.
Astatine-211: Meets Reality
Astatine-211 is capturing more and more attention for its near-perfect alpha emission profile and reduced off-target toxicity compared with actinium-225. Although it comes with significant challenges, its cleaner, shorter-lived, and theoretically safer characteristics makes it especially attractive for targeted alpha therapy (TAT).
However, as with Terbium, availability continues to limit its momentum. In Europe, current production is confined to a few specialized facilities in Denmark and France, with very limited global supply. Several academic and industrial groups are actively working to change this, but in the meantime, progress will depend on establishing reliable, scalable production. Astatine’s story is one of immense scientific promise that still needs logistical solutions to truly take off.
Actinium-225 Supply: A Step Forward
For all the talk of new isotopes, actinium-225 remains the backbone of alpha therapy programs, and this year brought good news. A key industrial supplier announced a major expansion of its production capacity, helping to ease what has long been a bottleneck for translational research.
While availability is still limited, these developments suggest that alpha-based radiopharmaceuticals could soon transition more smoothly from discovery to clinical evaluation. For developers planning upcoming studies, securing access early will continue to be a strategic priority.
From Monotherapy to Combination Therapy
Beyond isotopes, another noticeable trend was the shift toward combination strategies. Not long ago, targeted radiotherapy was often seen as a standalone treatment. Today, that mindset is shifting. A full session at EANM was dedicated to combinations of targeted radionuclide therapies (TRT) with other modalities, such as immune checkpoint inhibitors, PARP inhibitors, chemotherapy, or even other radiotherapies, to enhance efficacy while controlling toxicity. This reflects growing confidence in the safety and manageability of TRTs.
As radiopharmaceuticals become better understood, better tolerated and better handled, researchers are now exploring how radiation-induced DNA damage can be leveraged synergistically with other mechanisms. The early data are extremely promising and suggest this will be a major focus of the next generation trials.
Pretargeting: Complex but Promising
Talks on pretargeting approaches sparked genuine curiosity. The concept, where the targeting vector (for example, an antibody) is decoupled from the radioactive payload, offers a way to dramatically improve the therapeutic window for large, slow-clearing molecules.
In practice, this means first injecting a non-radioactive ligand that binds to the tumor, followed by a smaller radioactive molecule that “clicks” to the ligand already in place. The result: faster clearance from non-target tissues and higher tumor-to-background ratios.
It’s technically challenging, but feasible, and already progressing in clinical settings. Oncodesign Services has contributed to early-stage pretargeting studies now advancing into the clinic, proving that this innovative concept is more than theoretical. As the technology matures, it could become an important aspect for optimizing therapeutic index, especially for large biologics.
EANM 2025 showed a field in motion: technically challenging, but full of momentum. Terbium’s rise, Astatine’s promise, and the surge in combination and pretargeting approaches all point toward a more sophisticated and integrated view of radiopharmaceutical potential.