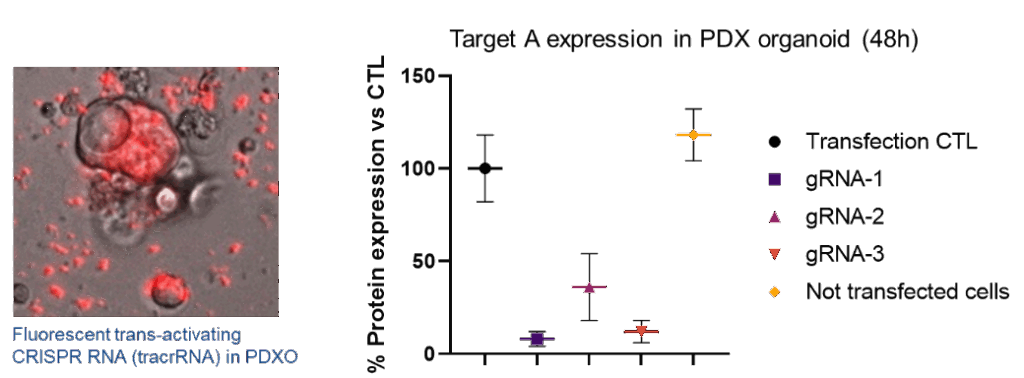


Cell-based assay services to support drug discovery and preclinical development
Cell-based assay services play a crucial role in modern drug discovery by offering a biologically relevant platform that closely mimics human cellular environments. Unlike biochemical assays, these assays preserve the intricate interactions between cellular components, as well as native substrates at physiological concentrations, allowing you to study drug candidates in a context that reflects real physiological conditions. The relevance of this data is essential for modeling disease states, elucidating mechanisms of action, and predicting therapeutic efficacy more accurately.
Key advantages
One of the major strengths of cell-based assays is their ability to capture complex cellular responses, including signal transduction, gene expression, and cellular metabolism. They are particularly valuable for toxicity assessment, early identification of off-target effects, and screening drug combinations for synergistic or antagonistic interactions. The scalability of these assays also enables high-throughput screening, accelerating the identification and optimization of lead compounds while contributing to the reduction of animal testing in early-phase research.
Cell-based reporter assays
Cell-based report assays, often created through precise cell engineering, offer additional advantages. By integrating reporter genes such as luciferase or GFP under the control of pathway-specific promoters, these cell-based reporter assays provide quantifiable and sensitive readouts of specific biological processes. This enables real-time monitoring of pathway activation or inhibition, making reporter assays especially useful in screening for modulators of transcription factors, receptor signaling, and cellular stress responses. The precision of engineered reporter cells enhances reproducibility and allows for robust comparison across different experimental conditions.
Phenotypic screening
Phenotypic screening, a prominent subset of cell-based assay services, further contributes to drug discovery by focusing on observable cellular outcomes rather than predefined targets. This unbiased approach enables the identification of novel mechanisms and drug candidates with complex modes of action, particularly valuable in areas like oncology, neurology, and infectious diseases.
Cell-based assays, including advanced reporter cell-based formats, are indispensable tools in the drug discovery toolkit. Their biological relevance, coupled with engineered precision and adaptability, not only enhances data quality and translational relevance but also drives more efficient and informed decision-making in your drug development pipeline.
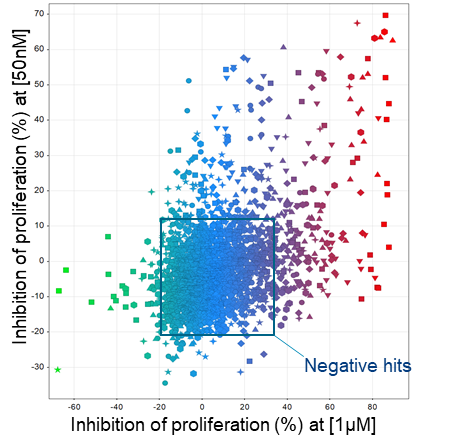
Above: Illustration of 2,500 compounds tested at 50nM and 1μM in a phenotypic screen. Each point depicted is one unique compound.
Oncodesign Services’ cell-based assay platform
Providing a direct link between in vitro and in vivo evaluation, Oncodesign Services’ platform enables selection of compounds and advancement into the best preclinical protocol. We provide experimental studies which determine:
- Cell viability, cell death, apoptosis, cytotoxicity, cell proliferation
- Compound induced cell resistance
- Cell migration and invasion
- Immune cells assays: Tumor cell interaction
- T cell proliferation, anergy
- PBMCs, T cells activation (IFNg, IL2 secretion, ELISPOT…)
- Dentritic cell activation, phagocytosis…
- Monocyte activation, macrophage polarization and phagocytosis, neutrophil phagocytosis
- T cells activation/signaling, macrophage activation
- Whole blood assay
- Clonogenicity
- ADCC/CDC
- Angiogenesis
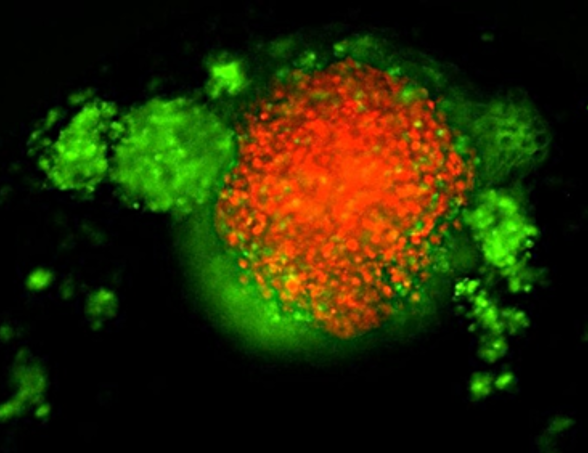
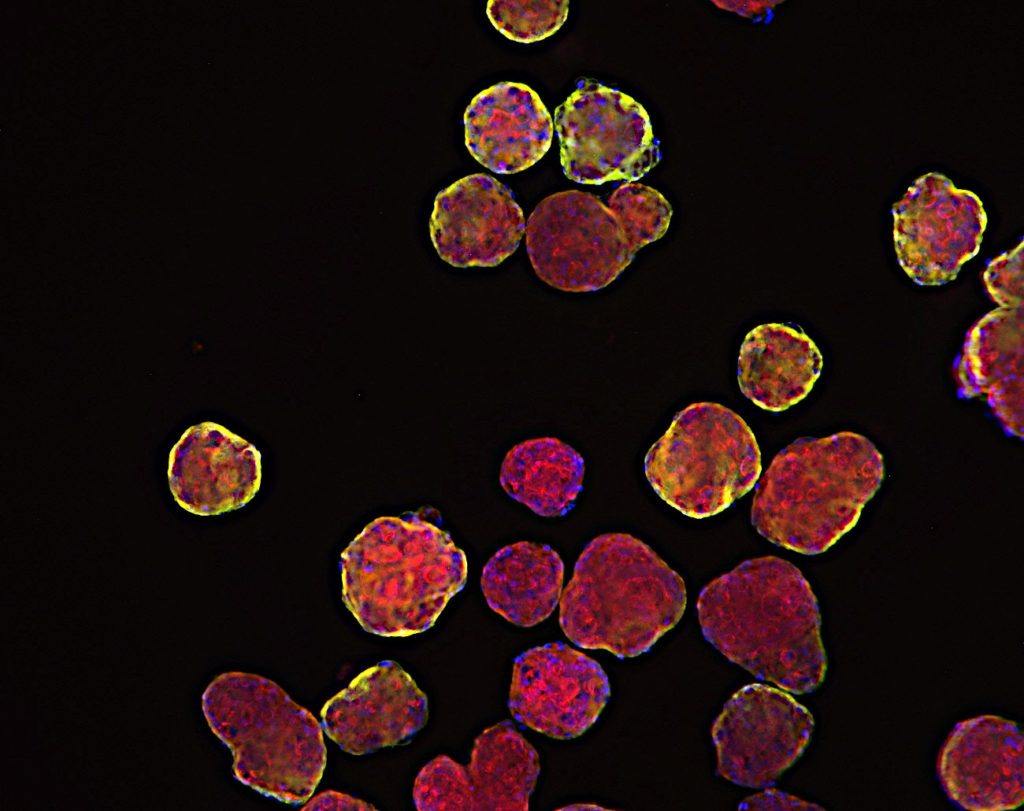
TNBC 3D spheroid expressing Kate2 (red) under attack by activated T cells (green)
A large panel of cell lines
To support our cell-based assay services, we offer a large tumor bank with >500 cell lines and primary cells, including coverage of 90% of human cancer. Cell-based assays can address tumor cell function, immune cell function or interaction of both cell types in a 2D or 3D format using a large variety of readouts. Tumor material can be sourced from cell lines or primary material of newly engineered models from various species. Immune cells can be sourced from healthy human donors, diseased donors, or animal models.
Cell engineering
We employ a wide range of molecular biology tools, such as CAS12-CRISPR-based techniques, fusion reporter gene design, and a broad selection of expression vectors to craft the optimal cell-based assay tailored to your specific requirements.
Cell engineering is essential in cell-based assays for drug discovery:
- Model diseases: Create disease-relevant cell lines for accurate testing.
- Validate targets: Confirm the role of drug targets.
- Signal amplification: Improve sensitivity with reporter genes.
- Personalized models: Develop patient-specific cell lines.
- Combat drug resistance: Study and address resistance mechanisms.
- Phenotypic screens: Simplify compound identification in phenotypic screens.
Our tailor-made, built-to-order cell-based reporter assays are designed to meet your specific research needs. Our portfolio includes classic pathways such as NF-κB, CRE/CREB, NFAT, ISRE, and SRE, each engineered with our proprietary enhancements to deliver improved signal-to-noise ratios and superior sensitivity. Whether you’re targeting well-characterized pathways or need a custom solution, our assays are optimized to provide reliable, high-quality data for your drug discovery program.
Our multi-disciplinary teams and dedicated labs offer the following facilities:
- BSL2 and BSL3 for pathogen handling
- Authorized for GMO use
- Authorized for human tissue derivatives use
- Registered biological resource center
- Radionuclide lab for in vitro assays
Example studies:
-
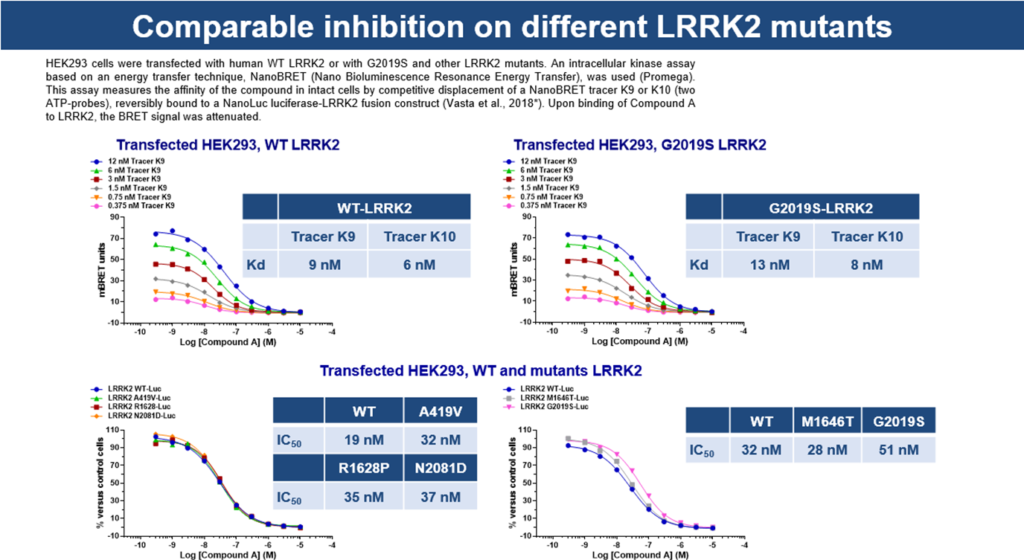
In-cell kinase target engagement assay
Intracellular assay on HEK293 cells transfected with human cDNA construct of WT LRRK2 or with G2019S and other LRRK2 mutants.
This assay measures the affinity of the compound in intact cells by competitive displacement of a NanoBRET tracer K9, reversibly bound to a NanoLuc luciferase-LRRK2 fusion construct. Upon binding of Compound A to LRRK2, the BRET signal was attenuated.
-
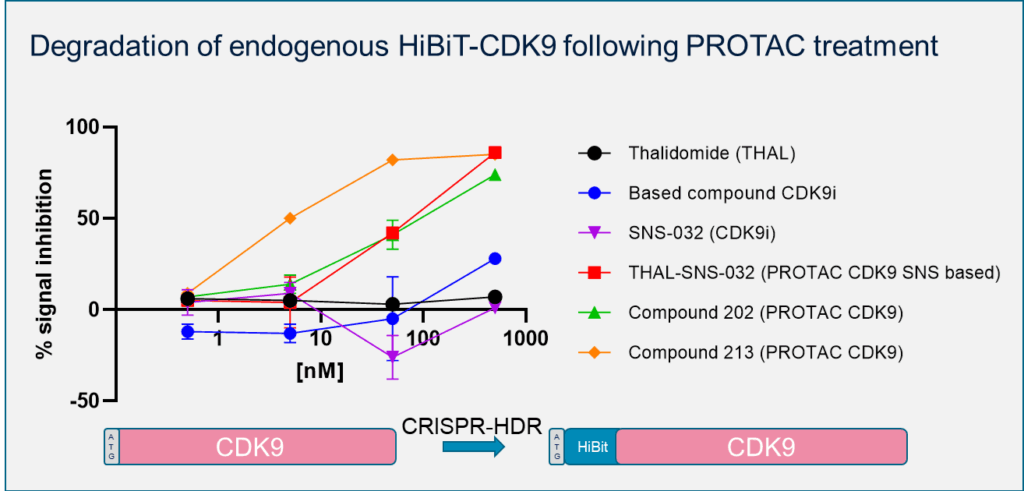
Degradation of endogenous HiBiT-CDK9 following PROTAC treatment
-
RNP lipofection of three gRNA in dissociated PDAC PDX-Organoid patient #83