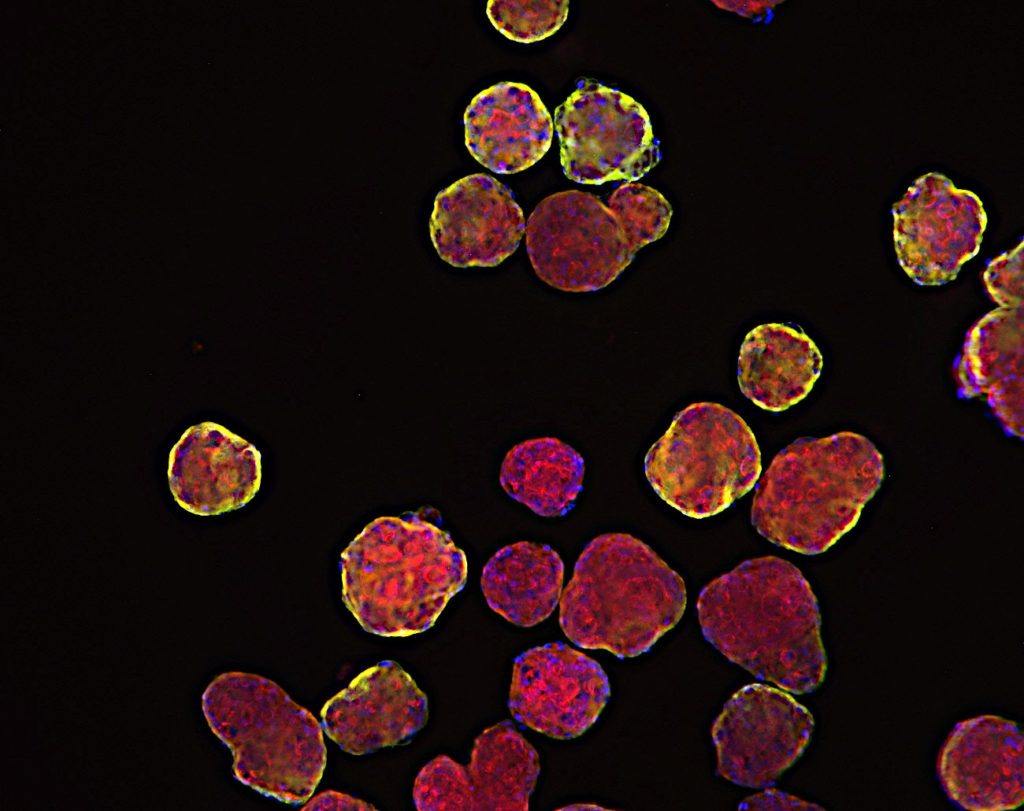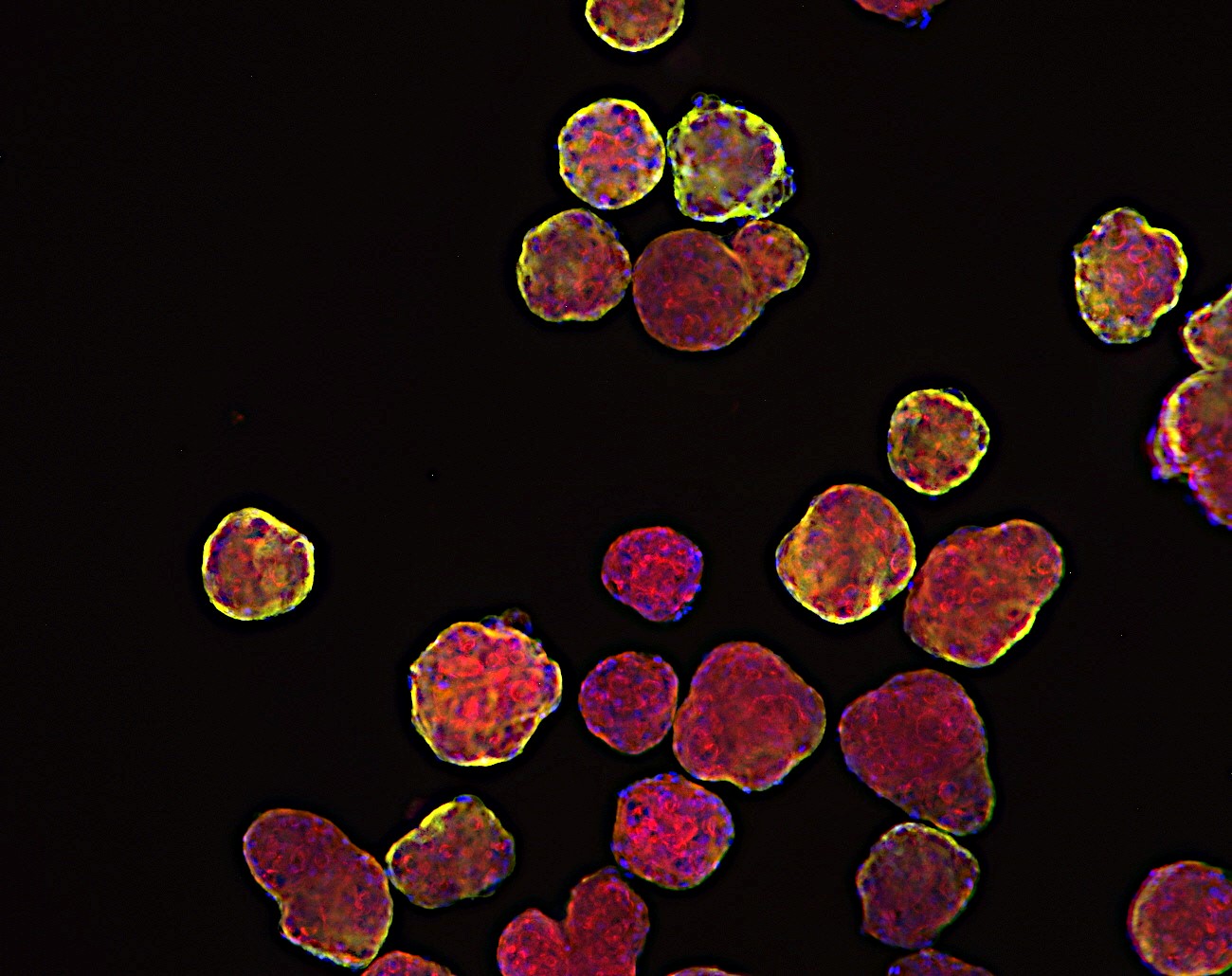


Breaking Barriers in Oncology Drug Development:
Unveiling the Potential of 3D Cell Models for Drug Discovery
In the ever-evolving landscape of oncology drug discovery, researchers are continuously seeking innovative approaches to bridge the gap between laboratory experiments and clinical success. One remarkable stride in this direction comes from the realm of 3D cell in vitro models, which includes micro tumoroids, organoids from tumors, spheroids from cell lines, and patient-derived xenograft (PDX) organoids. These advanced models are revolutionizing the way we understand, screen, and develop cancer treatments, offering a closer approximation to the complexity of human tumors.
A cornerstone advantage of these 3D cell models lies in their proximity to the intricate reality of patient tumors. By replicating the intricate cellular interactions and physiological conditions found within the human body, these models offer a higher likelihood of identifying drugs that will translate successfully to clinical trials. This alignment between lab findings and patient outcomes is a powerful driver in accelerating the drug development process.
Transforming cancer science with 3D cell models
Traditional 2D cell cultures have long been the standard in laboratory research, but they often fall short in replicating the complexity of in vivo environments. Enter cell lines derived spheroids, which are 3D cell aggregates derived from cell lines. Unlike 2D cultures, spheroids more accurately mimic cell-cell interactions and gradients of nutrients and oxygen found in tumors. This more realistic microenvironment enhances our understanding of drug penetration, toxicity, and efficacy, providing a better foundation for drug screening.
Micro tumoroids represent a groundbreaking advancement in mimicking the intricate environment of tumors within a lab setting. These tiny 3D structures are composed of a combination of cancer cells and supportive stromal cells. By recreating the microenvironment surrounding tumors, micro tumoroids allow researchers to observe how cancer cells interact with neighboring cells, extracellular matrix components, and blood vessels. This dynamic interaction offers valuable insights into tumor growth, invasion, and response to therapeutic agents.
Organoids derived from tumors take personalization to the next level. By culturing patient-specific tumor cells, these models preserve the unique genetic and molecular characteristics of individual cancers. Researchers can study the response of these tumor organoids to various drug compounds, helping to tailor treatments to a patient’s specific tumor profile. This approach holds immense promise for personalized medicine, where treatments can be customized to target the vulnerabilities of a patient’s tumor.
Among these models, patient-derived xenograft (PDX) organoids (PDXO) shine as an embodiment of translational potential. Merging the benefits of PDX models and organoid technology, these models retain the genetic diversity and complexity of tumors while enabling high-throughput drug testing. The breakthrough application involves the utilization of genetic manipulation in PDXOs, achieved by either enhancing the expression of specific targets or implementing gene knockdown through CRISPR technology. This approach not only deepens our understanding of individual patient responses to treatments but also facilitates in vivo studies using corresponding models, fostering a bridge between laboratory discoveries and clinical applications.
CRISPR technology is a revolutionary gene-editing tool that harnesses the precision of molecular biology to modify specific DNA sequences within an organism’s genome. In preclinical cancer drug discovery, CRISPR is utilized to create precise genetic modifications in cancer cells, allowing researchers to investigate the role of specific genes in cancer development and progression. By targeting and altering genes associated with cancer, scientists can gain valuable insights into potential drug targets, assess the efficacy of candidate drugs, and develop personalized therapies tailored to an individual’s genetic profile. This technology has dramatically accelerated the pace of cancer research and holds immense promise for the development of novel, targeted cancer treatments.
Among the multifaceted applications of 3D cell in vitro models, the utilization of PDXOs in phenotypic screens stands out as a transformative approach. Phenotypic screens involve observing the effects of potential drug candidates on the entire organism or, in this case, the 3D model of the tumor. This comprehensive approach not only aids in identifying potential drug leads but also serves as a critical step in refining chemistry for lead optimization and unraveling new targets.
Beyond their clinical potential, the integration of 3D cell models also presents ethical and practical advantages. These models substantially reduce the necessity for animal experimentation, aligning with ethical research practices and expediting drug development by eliminating less relevant layers of experimentation.
Additionally, the abundance of experimental material facilitated by 3D cell models is a significant benefit. These models also allow for genetic manipulation, enabling the exploration of molecular mechanisms underlying drug responses, paving the way for novel therapeutic discoveries.
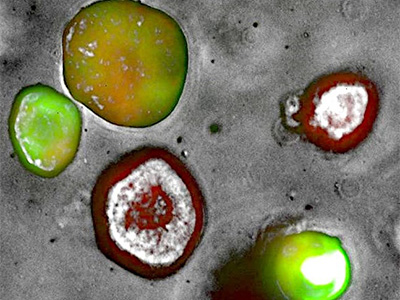
PDAC PDX-Organoid stably transfected using a tri-cistronic engineered vector expressing mCherry-EGFP-NanoLuc
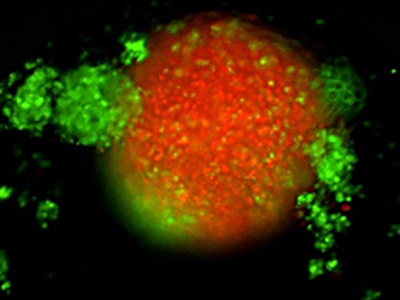
TNBC 3D spheroid expressing Kate2 (red) under attack by activated T cells (green)
Robust Preclinical Data for In Vitro Proof of Concept
At Oncodesign Services, we’ve successfully prepared numerous PDXO models and subjected them to various manipulations, such as drug treatments, drug combinations, and precise genome editing using CRISPR or lentivirus techniques. These endeavors have significantly enhanced our comprehension of the intricate connections between therapeutic targets and their mechanisms of action, as well as providing invaluable in vitro proof of concept. These streamlined processes are particularly advantageous for expediting the design-make-test cycle within our integrated team, which encompasses chemistry, DMPK (Drug Metabolism and Pharmacokinetics), and in vitro biology experts. Moreover, the same PDX models can be further assessed to validate their in vivo pharmacological properties, ensuring comprehensive preclinical insights.
In conclusion, the paradigm shift brought about by 3D cell in vitro models is reshaping the landscape of oncology drug discovery. From micro tumoroids and tumor organoids to PDX-derived organoids, these models provide a closer representation of patient reality, reducing the reliance on animal models, offering more experimental material, and enabling genetic manipulations. As we move forward, it’s clear that these 3D models will continue to play a pivotal role in accelerating the development of effective cancer therapies, ultimately translating into better outcomes for patients battling this formidable disease.
About the author
This blog post was written by Dr Nicolas Ancellin, strategic project director at Oncodesign Services. He is an experienced cell and molecular biologist specializing in drug discovery. In his current role, Nicolas is involved in Explore, our offering designed to support identification and validation of therapeutic targets.