

Unique preclinical solution for theranostic drug development, from bench to bedside
At Oncodesign Services, we support biotechs and pharma in accelerating the discovery and preclinical development of innovative theranostic compounds. By combining expertise in oncology pharmacology, radiopharmaceutical chemistry and nuclear medicine, we act as a theranostic CRO. Our integrated approach bridges diagnostic and therapy.
What are ‘theranostics’?
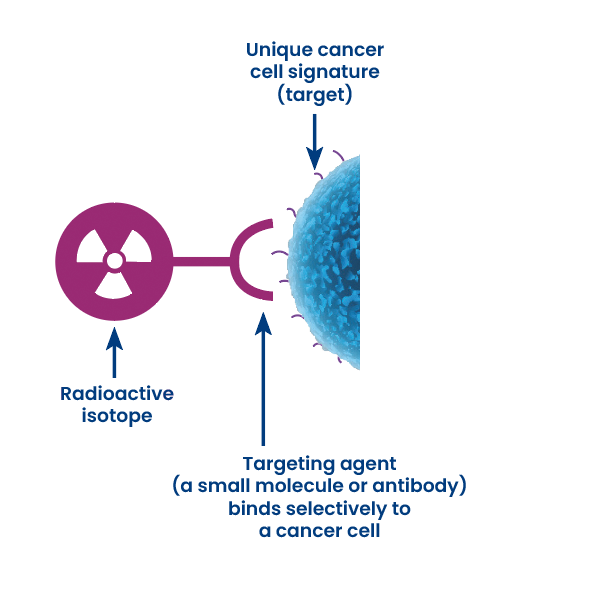
In the field of nuclear medicine, theranostics often involves the use of radiopharmaceuticals, compounds that contain a radioactive isotope, is designed to have both diagnostics and therapeutic properties.
Example of theranostics in oncology : biomarkers
One example of radiopharmaceutical theranostics is in the field of oncology. Specific biomarkers present in a patient’s tumor can be identified by diagnostic tests such as pharmaco-imaging tools where the biomarker is conjugated to a γ (gamma) or β+(positron) emmitter radionuclide (SPECT or PET scan, respectively). The same biomarkers, can be used for personalized treatment when a β– (beta), α (alpha) or auger emitter radionuclide is conjugated and we can provide a personalized targeted treatments options (I.e radioimmunotherapies, molecular targeted therapies) relevant to the molecular properties of the tumor. Moreover, we can monitoring the response to treatment using the imaging theranostic counterpart allowing a for timely adjustments to the therapeutic scheme , if necessary.
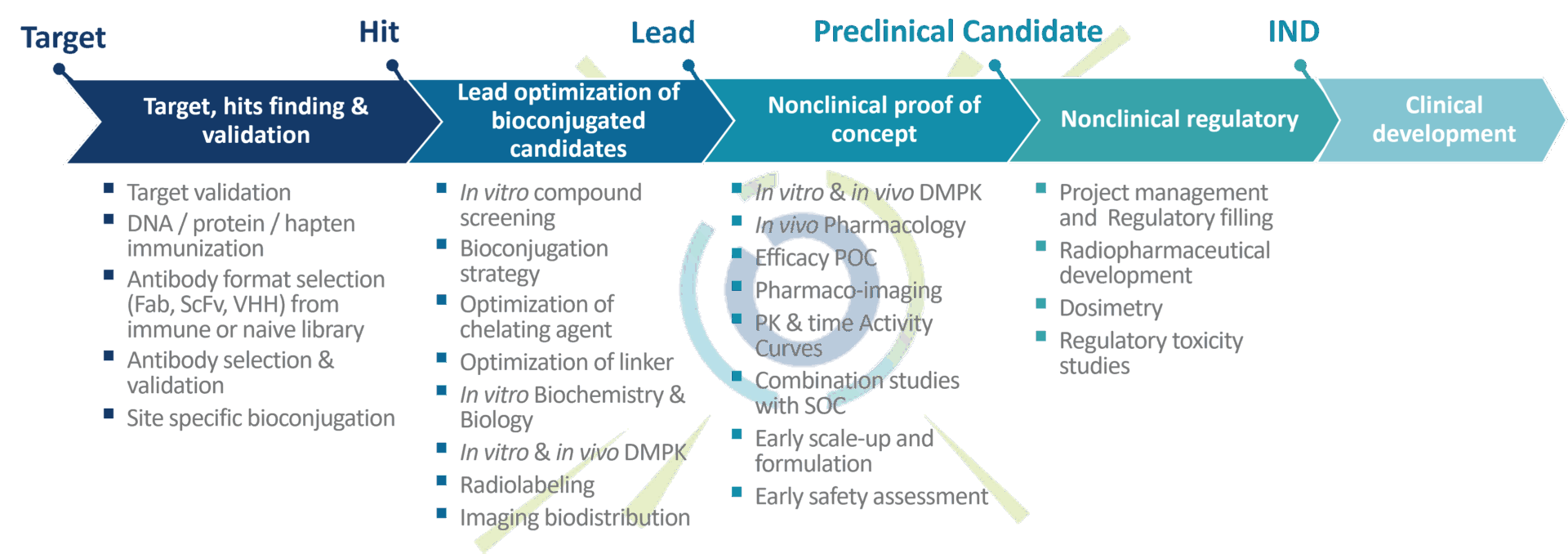
Oncodesign Services offers integrated solutions for theranostics approach
The development of theranostics requires highly specific expertise and strong collaboration between preclinical and clinical development. Oncodesign Services offers a unique service solution for theranostic drug development, from bench to bedside.
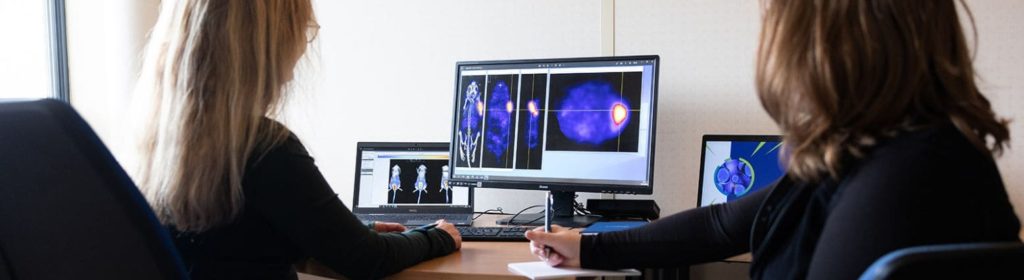
A strong imaging platform to understand the pharmacology of the theranostic:
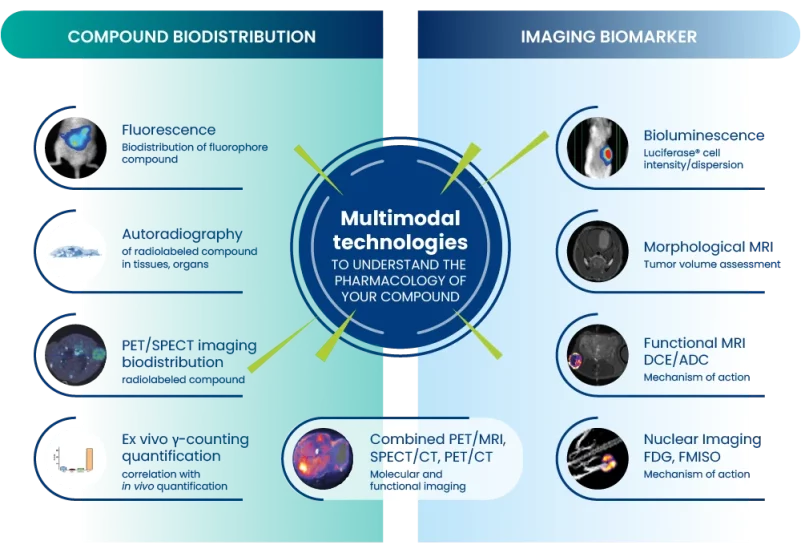
Oncodesign Services enables faster and cost-optimized development combining radiopharmaceutical development, molecular imaging and preclinical oncology. We have built a value proposition in which the generation of radiolabeled biological vectors, chelator production, design of the bioconjugated lead, preclinical proof of concept and the design of the regulatory package are managed effectively and efficiently.