

- Protection of RIPK2 inhibitors resulting from Nanocyclix® technology with patents granted on the main international markets: Europe, United States, Japan and Eurasia
- Preclinical toxicological evaluation of the RIPK2 drug candidate ODS-101 successfully completed
- A solid patent portfolio with 104 applications approved or currently being assessed concerning Oncodesign’s proprietary kinase inhibitors
Paris and Dijon (France), November 4, 2021 at 6:00 pm CET – ONCODESIGN (ALONC – FR0011766229), a biopharmaceutical group providing the pharmaceutical industry with support finding new therapeutic molecules for fighting various forms of cancer and other serious illnesses with no known effective treatment, announces the strengthening of its patent portfolio concerning RIPK2 (Receptor-interacting serine/threonine-protein kinase 2) inhibitor macrocyclic compounds, as well as the successful completion of the preclinical toxicological evaluation of its ODS-101 drug candidate.
The RIPK2 kinase inhibitors developed by Oncodesign have substantial therapeutic potential in inflammatory intestinal diseases aiming for a specific targeted action, a market estimated at close to 7 billion US dollars in 2023, an increase of +17% compared with 2018 (global figures). The medical need for an orally administered immune response regulator in inflammatory bowel disease is substantial, and complements current therapeutic options based on immunosuppressors and monoclonal antibodies.
Jan Hoflack, Oncodesign’s Chief Scientific Officer, commented: “The granting of a patent is essential for protecting the innovation created by Oncodesign’s research teams. The development of our portfolio of patents covering our RIPK2 inhibitors, and on which we have the exclusive rights of use, is crucial in highlighting the value of this program to a partner for the development of our drug candidate”.
The approval in India of the initial patent (WO2016/042087 A1) covering the RIPK2 inhibitors resulting from Nanocyclix® technology, for which Oncodesign has the exclusive rights of use, further strengthens the patent family it is a part of that covers the main international markets such as Europe, the United States, Japan and Eurasia. This patent is valid until September 2034. Furthermore, a selection patent (WO2021/152165 A1), concerning the latest generation of RIPK2 inhibitors resulting from Oncodesign’s research, was published in August 2021, also contributing to this strengthening of the intellectual property covering the drug candidate and its followers[1].
The preclinical toxicological evaluation of the RIPK2 drug candidate ODS-101 has been successfully completed. The teams are now preparing the filing of the regulatory dossier (CTA – clinical trial application) in order to obtain approval from the ANSM (the French Agency for the Safety of Health Products) to undertake a Phase 1 clinical study in healthy volunteers (first in human administration) in 2022.
Oncodesign is thus continuing the development of its intellectual property concerning the proprietary molecules resulting from its Nanocyclix® technology and their associated targets. Oncodesign currently has a patent portfolio of 104 applications either approved or being examined, protecting the molecules developed within the framework of Oncodesign’s proprietary programs.
[1] Followers are molecules with the same activity on RIPK2 that belong to the same chemical class
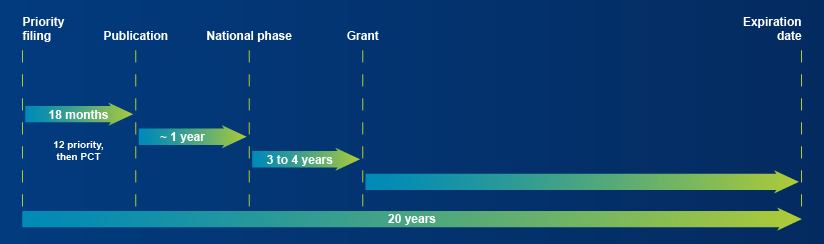
Patent life cycle
About Oncodesign
Oncodesign is a biopharmaceutical company dedicated to precision medicine, founded in 1995 by its current CEO and majority shareholder, and has been listed on Euronext Growth Market since April 2014. Its mission is the discovery of effective therapies to fight cancer and other diseases without therapeutic solutions. With its unique experience acquired by working with more than 1 000 clients, including the world’s largest pharmaceutical companies, along with its unique technological platform combining Artificial Intelligence, state-of-the-art medicinal chemistry, pharmacology, regulated bioanalysis, medical imaging, Oncodesign is able to select new therapeutic targets, design and develop potential preclinical candidates through to clinical phases. Oncodesign has configured its organization to offer innovative services to its customers and to license its proprietary molecules. Applied to kinase inhibitors, which represent a market estimated at over $65 billion by 2027 and accounting for almost 25% of the pharmaceutical industry’s R&D expenditure, Oncodesign’s technology has already enabled the targeting of several promising molecules with substantial therapeutic potential, in oncology and elsewhere, along with partnerships with global pharmaceutical groups. Oncodesign is based in Dijon, France, in the heart of the town’s university and hospital hub, and within the Paris-Saclay cluster. Oncodesign has 233 employees within 3 Business Units (BU): Service, Biotech, Artificial Intelligence and subsidiaries in Canada and the USA.
Contacts Press
Oncodesign |Philippe Genne | CEO
Tel. : +33 (0)380 788 260
NewCap | Investor Relations | Mathilde Bohin / Louis-Victor Delouvrier
Tel. : +33 (0)144 719 495
NewCap | Media Relations | Arthur Rouillé
Tel. : +33 (0)144 710 015
Disclaimer
This press release contains certain forward – looking statements and estimates concerning the Company’s financial condition, operating results, strategy, projects and future performance and the markets in which it operates. Such forward-looking statements and estimates may be identified by words such as “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions. They incorporate all topics that are not historical facts. Forward looking statements, forecasts and estimates are based on management’s current assumptions and assessment of risks, uncertainties and other factors, known and unknown, which were deemed to be reasonable at the time they were made but which may turn out to be incorrect. Events and outcomes are difficult to predict and depend on factors beyond the Company’s control. Consequently, the actual results, financial condition, performances and/or achievements of the Company or of the industry may turn out to differ materially from the future results, performances or achievements expressed or implied by these statements, forecasts and estimates. Owing to these uncertainties, no representation is made as to the correctness or fairness of these forward-looking statements, forecasts and estimates. Furthermore, forward-looking statements, forecasts and estimates speak only as of the date on which they are made, and the Company undertakes no obligation to update or revise any of them, whether as a result of new information, future events or otherwise, except as required by law.
