

Preclinical Services to support your biologics development and preclinical testing
What are biologics?
New biological entities or biological therapeutic products (namely Biologics) include a huge diversity of products such as : vaccines, gene and cellular therapies, recombinant therapeutic proteins, naked or conjugated monoclonal antibodies, bispecific antibody-like structure and others. Biologics can be composed of sugars, proteins, nucleic acids or complex combinations of these substances, or may even be living entities.
Biologics made a first revolution in cancer treatment with approval of rituximab and trastuzumab in late 1990’s (two monoclonal antibodies targeting antigens expressed on tumor cells). A second major revolution was brought in the early 2010’s with the approval of antibodies that target immune checkpoint on immune cells (i.e. ipilimumab targeting CTLA-4 positive regulatory CD4 T cells and nivolumab or pembrolizumab, both targeting PD-1 on T cells) rather than tumor cells. Since 2017, there have been about 10 to 15 biologics approved each year, and much more entering clinical development, which represents a rapidly growing market in various therapeutic areas such as oncology, autoimmune diseases, inflammation, infectious diseases and others.
Successful R&D requires pharma, biotech and startups, contract research and technological based companies to cooperate in a synergistic fashion, each partner bringing its scientific contribution and technical expertise Building the right internal and external partnerships helps de-risking a drug development program
Oncodesign Services’ biologics CRO solution offering
Oncodesign Services provides a unique integrated solution from biologics discovery to clinical and commercial manufacturing. In oncology and inflammatory diseases, we support companies to develop and test naked and bioconjugated antibodies and derivatives.
-
Ligand Binding Assays Platform
- Immuno-analysis / LBA / CBA / (RT)qPCR / dPCR
- Immunogenicity assessment:
-
- ADA (Anti-Drug Antibodies) : screening, confirmatory and neutralizing assays
- Cytokine measurements
- Immunophenotyping
-
Translational pharmacology platform
- In vitro & in vivo
- Target expression
- Binding, specificity, mechanism of action (direct effect, immune mediated effect)
- Efficacy models in oncology (CDX, PDX, humanized), inflammation, microbiome, infectious diseases
-
PK/PD and biodistribution
- Set-up of specific assays (ELISA, MS….)
- Bioanalysis (non-GLP, GLP and GCP)
- PK/PD in healthy, tumor bearing rodent and FcRn mice
-
Molecular imaging and molecular radiotherapy
- Bioconjugation and radiolabeling
- Binding assessment
- Biodistribution (nuclear imaging) efficacy
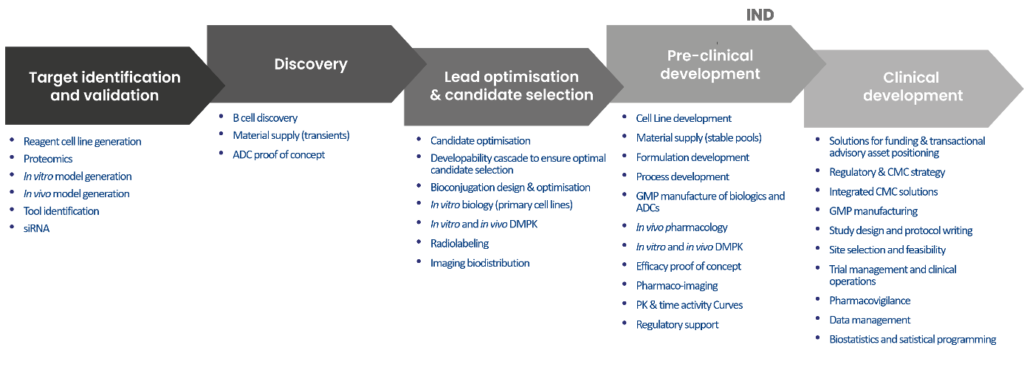
Discover DRIVE-Biologics: our specific offer for biologics development and preclinical services
The DRIVE Biologics consortium provides a unique integrated solution of specialist services with strategic partners. These services enable to design, optimize and develop novel biological entities addressing the therapeutic target of interest. DRIVE Biologics supplies the high level, IND focused discipline to rigorously manage the integrated programs from the early stages of discovery through to preclinical development, CMC, manufacturing, regulatory affairs and clinical trials
A strong alliance of experts
 |
 |
 |
