

Access a large collection of PDX mouse models for in vivo pharmacology studies
Patient derived xenograft mouse models (PDX) are models developed for cancer where the tissue or cells are implanted into an immunodeficient or humanized mouse directly from a patient’s tumor. In translational services, PDX models allows us to recreate a more human environment in which we can study cancer progression, and the efficacy of new oncology therapies.
With the support of a CRO (Contract Research Organization) partner, PDX mouse models give you high quality data and predictive information of how the new therapic will perform in human, before entering into clinical trials.
Why use a PDX Mouse Model?
In drug discovery, one of the major factors is the need for cancer models that better mimic the native tumor diversity and microenvironment and thus respond to potential cancer drugs in a manner more representative of a human response.
The Patient derived xenograft mouse models (PDX) appeared as a reliable option for:
- Mimic human tumor microenvironments: It maintains patient tumor architecture, making it more predictive than traditional xenografts.
- Support biomarker and resistance studies: ideal for identifying predictive markers, stratifying patient populations, and studying acquired resistance in both oncology and immuno-inflammatory pathways.
- Enables robust preclinical trials: PDX cohorts (also known as mouse preclinical trials SMPT) yield high quality, translational data to inform therapeutic decisions early.
We offer a comprehensive collection of PDX models (target organs: liver, breast, lung, brain, colon, pancreas…). These PDX models have been characterized in regard to:
- Histology
- Conservation of phenotype and genotype
- Gene expression stability
- Gene mutation status
- Response to standard of care (SOC)
If the appropriate model is not available, we can develop a customized version to your project needs.
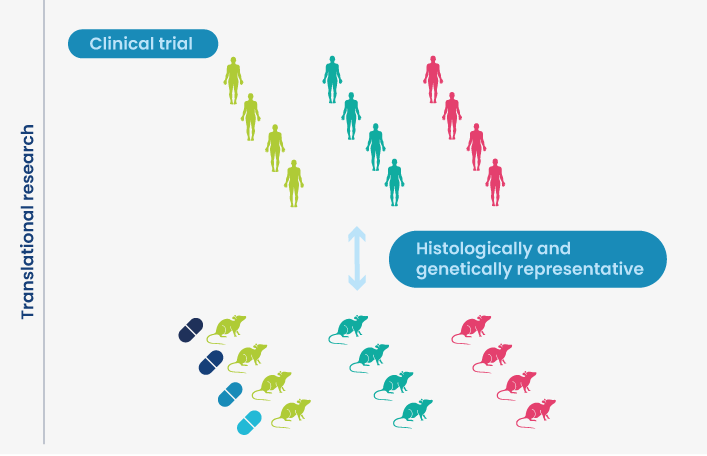
Single Mouse Preclinical Trial (SMPT): an in vivo design allowing screening of PDX models
Patient-derived xenograft (PDX) mouse models are considered the gold standard for translational in vivo tumor modeling, yet they are slow to grow and not easily scaled up. There is considerable market demand for a solution that combines the translatability of PDX tumors and the cost-effectiveness of in vitro screening.
The SMPT format employs a single mouse* per PDX model and treatment arm, thereby enabling a cost-effective investigation of in vivo efficacy in large panels of PDX models. This type of experiment is recapitulating the clinical situation with identification of responders versus non responders to treatment.
*possible to increase slightly this number.
The SMPT is designed for drug developers who need to characterize:
- the range of responders accross many indications
- the signature of response of drugs
- how responders change with drug combinations
Do you need PDX models for your research programs? Contact us!