
Challenges and perspectives in the preclinical development of therapeutic agents against lung fibrosis


Pulmonary fibrosis is estimated to affect more than 5 per 100,000 people per year in the USA and Europe. It can lead to chronic respiratory insufficiency. Very rare before the age of 50, this disease affects the older population, and is often diagnosed at an advanced stage. Furthermore, limited treatment options are available to those suffering from it. There is therefore a major medical need to find new drugs able to slow down, stop or even reverse the progression of the disease. Here, we examine the current knowledge about pulmonary fibrosis, and preclinical options available to develop new therapeutic solutions.
Fibrosis is a condition in which the organs become scarred over time. It is the consequence of chronic fibroblast and myofibroblast activation.
Some associated risk factors have been identified such as exposure/irritation (tobacco, dust, …) /infection of the respiratory system. These aggressions would be responsible for the triggering of a dysregulated scarring process.
However, in 20 to 25% of affected patients, fibrosis occurs without any identified cause, and is therefore called idiopathic pulmonary fibrosis.
Idiopathic pulmonary fibrosis is a progressive and fatal disease, with nevertheless a diversity of evolution across patients: in some, the disease remains stable over the course of several years (3-6 years), while in others, the disease worsens, leading to the patient’s death within a year from diagnosis. The estimated survival rate at 5 years does not exceed 20%.
The only treatment currently able to improve survival is lung transplantation. It is offered to people under 65 years of age at an advanced stage of the disease. Symptom-attenuating measures can also be implemented, such as oxygen therapy and respiratory rehabilitation. However, their interest only lies in the partial easing of the patients’ discomfort, rather than disease reversion, thus not causing any survival improvement. Besides, beyond a certain point, severe lung function loss can no longer be compensated.
The anti-cancer agent bleomycin as well as radiation therapy for lung cancer are known to lead to the development of pulmonary fibrosis in most patients. In both cases, fibrosis is secondary to an inflammatory phase.
As for idiopathic pulmonary fibrosis, in both cases the fibrosis that develops constitutes a chronic progressive disease, crippling patients due to ventilatory functions deterioration.
The reference triple therapy for idiopathic pulmonary fibrosis is based on the administration of:
with the aim of reducing the inflammatory response to prevent the spread of scar tissue in the lungs and slow the progression of the disease. Recently, a new small molecule, Nintedanib*, an inhibitor of non-receptor tyrosine kinases, has been released on the American and European market.
Still, these treatments merely slow the progression of the disease, but fail to stop or reverse it. Besides, their side effects can sometimes cause the patient to spontaneously discontinue treatment or justify the medical decision to stop the medication.
Given the limited therapeutic arsenal to tackle lung fibrosis, preclinical research remains a necessity to provide robust animals models recapitulating – at least partially – the features of the human disease.
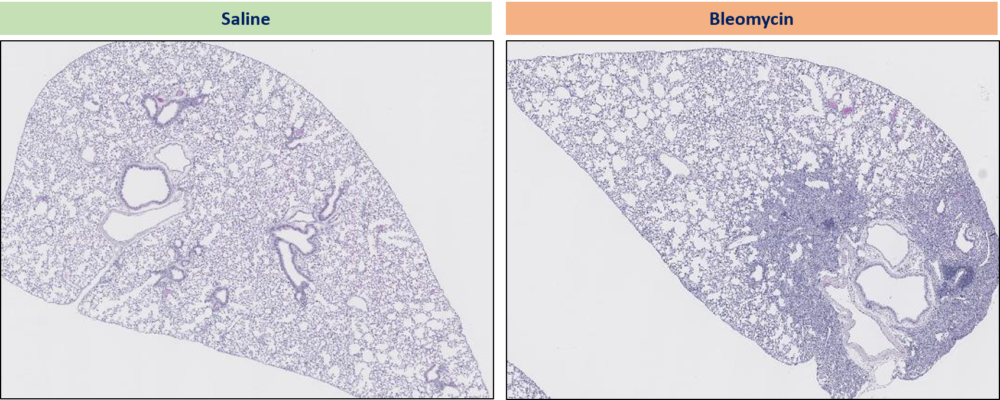
Specifically, the deleterious profibrotic effect of bleomycin or radiation can be taken advantage of in mice and rats. In practice, bleomycin, delivered locally, induces pulmonary fibrosis in 3 weeks in rodents. Radiation therapy leads to pulmonary fibrosis in 14 weeks in rodents.
Test drugs can then be administered in the fibrotic rodents by any relevant route, in a prophylactic or therapeutic pattern. Nintedanib is typically used as the positive control, in order to compare the efficacy of new drug candidates to this partially effective small molecule.
Critically, in order to provide a reliable evaluation of the beneficial effect of any treatment on lung fibrosis, and to predict potential efficacy in humans, lung functional evaluation must be performed. Automated forced ventilation systems have now been developed to passively perform such measures in deeply anesthetized rodents. Such specialized devices can blow air into the lungs, mimicking a forced inspiration, or suck it in, mimicking a forced expiration, while measuring the pressure generated.
In the clinic, plethysmography is the measurement of lung function by evaluating mechanical parameters of the lung tissue. It is used to evaluate disease severity and to provide a prognosis. The most relevant parameters are lung capacity (volume that can be contained by the lungs), airflow (amount of air inhaled and exhaled), and lung compliance (flexibility of the lung tissue, i.e. ability to deform according to the amount of air).
At Oncodesign Services, we have strong experience with several lung fibrosis models, and have implemented and validated the flexiVent® system (Scireq™). This system provides clinically relevant readouts of lung function. Combining of a variety of readouts allows us to provide robust preclinical models of lung fibrosis. These readouts include clinical scoring of animal health, lung imaging (CT-scan), histology, lung functionality and gene expression. This approach allows to evaluate the efficacy of our client’s candidate drugs along all relevant dimensions of the disease.
If you would like to know more about inflammation studies and in particular fibrosis studies, and how Oncodesign Services can help your projets, contact our team via the contact form below.
This article was written by Pauline Bornert, PhD, DVM. In her current role at Oncodesign Services, Pauline is involved in inflammatory, auto-immune and infectious diseases R&D programs for customers.
*FDA approves first treatment for group of Progressive Interstitial Lung Diseases, 09 March 2020, https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-group-progressive-interstitial-lung-diseases
Do you want to know more on our Inflammation capabilities ? Contact-us !