
Efficacy study insights from a leading CRO to evaluate the impact of novel therapies
To better evaluate the efficacy of new therapies, Oncodesign Services has developed specific approaches and models (ex vivo/in vivo) to conduct efficacy POC studies.
What is a efficacy study in pharmacology?
In pharmacology, efficacy refers to the ability of a drug or a compound to provide a beneficial therapeutic effect. Efficacy studies aim to determine how well a new treatment works under controlled conditions, and whether it provides the intended benefits.
A crucial parameter in drug development, efficacy is the basis of strategic decision-making in preclinical and clinical trials.
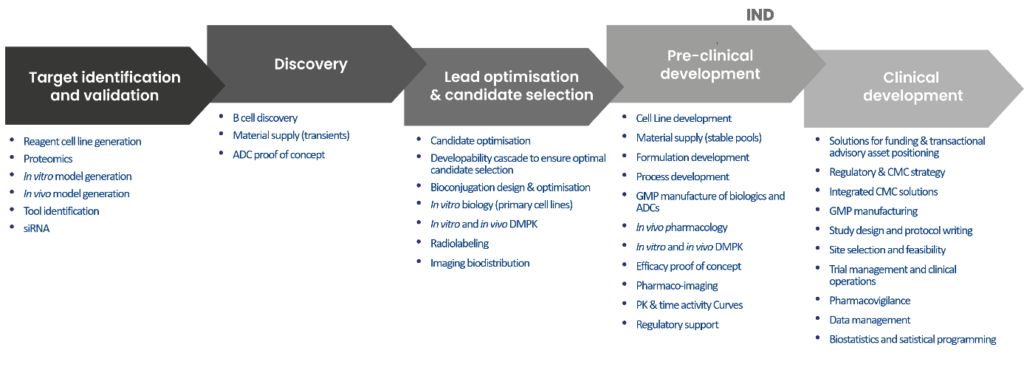
What are the key components of efficacy studies ?
An efficacy study is composed of several steps :
- Study design : The objectives of efficacy study are designed. It is important to customize the study plan to meet specific client needs, from the sampling method, the study duration and the type of investigation.
- Model selection: The models used for studying diseases must exhibit pathological similarities closely resembling the conditions observed in humans. Experimental animal models are therefore useful to understand disease mechanisms better and to evaluate the therapeutic efficacy of new and emerging drugs.
- Regulatory compliance: All studies must be conducted under the approval of ethics committes and regulatory bodies.
Why choose Oncodesign Services for your efficacy studies?
Oncodesign Services provide expertise and technical resources to ensure high-quality efficacy studies. We combine outstanding scientific knowledge, an in-depth expertise of the model, state-of-the-art techniques, relevant clinical endpoints with GLP-like data analyses, SOPs and reporting. We can also develop custom-made efficacy models.
We choose cutting-edge technologies such as non-invasive imaging tools to generate optimal quantifications of your preferred efficacy endpoints (tumor growth, infection progression…), ensuring accurate measurements and improving the translatability of your preclinical results to human studies.
Our validated models apply for a variety of therapeutic areas, including oncology, the cardiovascular domain, hemostasis, and the respiratory domain.
We are committed in providing high-quality data and reports that help you make informed decisions for the development of your drugs!

