The Advantages of an Integrated Hit-to-Lead Process in Drug discovery
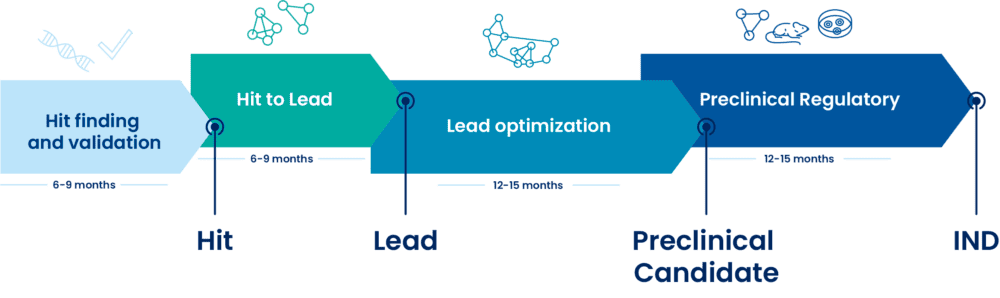
Developing an innovative therapy needs to build supporting evidences about the efficacy of the new molecule. For that, pharmaceutical companies must respect key preclinical stages, including a hit-to-lead process. The goal of the Hit-to-Lead (H2L) phase is to identify – after rational design and selection based on multiple-parameter analysis – the most promising Lead series to undergo a Lead-to-Candidate (L2C), also known as ‘lead optimization’ phase.
Oncodesign Services, a leading CRO in early drug discovery, guides you to success with an integrated hit-to-lead services.
Hit-to-lead process in early drug discovery
What is a Hit?
In the drug discovery process, a Hit is a compound that displays desired biological activity towards a drug target and reproduces this activity when retested.
There are several methods for Hit finding such as High-Throughput Screening (HTS), virtual screening (VS), or fragment-based drug discovery (FBDD).
- HTS involves testing a large number of compounds against a target protein (biochemical screening) or a cell-based (phenotypic screening) assay to identify those that bind or modulate the activity. This is typically done using automated techniques that can test thousands of compounds in a short period of time.
- Virtual screening involves using computational techniques, such as molecular docking and molecular dynamics simulations, to predict which compounds within a large library are likely to bind to a target protein.
- In the Fragment-based drug discovery (FBDD) approach small fragments of molecules are identified that bind to a target protein and subsequently function/act as a starting point to design more potent and selective compounds by growing the fragment or linking together different fragments.
When a suitable Hit is selected, it is engaged into several iterative DMTA (Design-Make-Test-Analyze) cycles in order to improve its potency, selectivity and early ADME properties to become a Lead.
What is a lead ?
A lead is a chemical compound within a defined chemical series having demonstrated a robust pharmacological and biological activity on a specific therapeutic target. This chemical structure is used as a starting point but require further optimization through chemical modifications or structural changes, in order to improve its efficacy, selectivity, pharmacokinetic properties, and safety profile.
For that, the Lead Optimization (LO) phase aims to improve the pharmacological profile and properties of the Lead compounds, such as efficacy, safety, metabolism, pharmacokinetics and pharmacodynamics, to make it suitable for development into a drug. The LO process includes chemical modification, structure-activity relationship (SAR) studies, and biological testing in relevant animal models.
What is the Hit-To-Lead Process?
The Hit-to-Lead (H2L) phase is about establishing quickly and rigorously the understanding of the Structure-Activity-Relationships (SAR) of different hit series. From a Hit finding campaign which identified several hit series, Hit qualification and characterization is carried out by various orthogonal methods, including biophysical, biocellular, and ADME assays, in order to recommend the most promising Hit series.
Depending on the clustering of the Hit series identified in the screening step, Hit-to-lead (H2L) is usually initiated on a minimum of 3-4 different chemotypes, leading to the selection of at least 2 series for the Lead-to-Candidate (L2C), or Lead Optimization (LO) phase. We recommend generating back-up Lead series as it increases the probability of success and reduces attrition rates, especially in the expensive downstream preclinical development phases.
The major objective at the end of Hit-to-Lead (H2L) step is to provide a comparative assessment and evaluation of all hit series, in order to select the most promising chemically different series with drug-like properties to undergo subsequent Lead Optimization phase.
An accelerated Hit-to-lead (H2L) phase can take on average 6-9 months, designed to efficiently validate and select drug-like series for the Lead-to-Candidate (L2C) phase, by reaching the desired criteria. The Lead criteria will be established from a Candidate Drug Target Profile (CDTP) defined in collaboration with the Sponsor.
Oncodesign Services offers integrated hit-to-lead services
Oncodesign Services is a contract research organization (CRO) with a mission to contribute to the discovery of novel therapeutics agents with high unmet medical needs on behalf of its clients.
At Oncodesign Services, we approach the Drug Discovery process with a clear vision of the desired outcome in mind. This includes considering factors such as the intended use, recommended dosage regimen and frequency, method of administration, necessary safety and effectiveness requirements defining the Target Product Profile (TPP). Those considerations shape the overall strategy for early drug development. The strategy is then translated into tangible elements and metrics in the Candidate Drug Target Profile (CDTP), aligning with the objective of the program and portfolio.
Oncodesign Services commits to execute the Hit-to-lead (H2L) processes in a Multi-Parameter Optimization (MPO) model and applies an “immediate processing” methodology, where compounds are processed into the screening cascade automatically, translating into fast DMTA (“Design-Make-Test-Analyze”) iterative cycles of optimization.
Our Hit-to-lead (H2L) services include:
- Biophysics SPR, DSF, ITC, NMR
- In vitro biochemistry & biology
- In vitro and in vivo DMPK
- Early safety assessment
Discover DRIVE-SM, an integrated drug discovery solution for small molecule
DRIVE-SM offers a complete solution through the entire small molecule drug discovery value chain from hit-finding, hit to lead, lead optimization, preclinical candidate through to IND-filing, aiming to deliver and accelerate the entry of high quality new chemical entities into the clinic.
Learn more on our drug development services? Contact us!