Dosimetry CRO services to support IND enabling preclinical and clinical development
Before conducting clinical trials, a comprehensive toxicology package is mandatory by regulatory authorities to be conducted. For novel diagnostic and therapeutic radiopharmaceuticals, the toxicity to healthy tissues and organs due to radiation is an additional aspect that should be addressed.
Dosimetry is employed to extrapolate findings from in vivo studies to predict potential radiotoxicity to human tissues and organs at risk and determined the limits of the administered radiation dose. By considering difference in factors such as body weight, physiology, and drug clearance rates from animals to humans, dosimetry is extrapolated from mice to human, assisting in the estimation of the appropriate starting doses for clinical trials.
Indeed, dosimetry plays an essential role in drug discovery and provides valuable throughput at the various stages of drug development from bench to bedside:
Radiation dosimetry helps in evaluating the potential risks and safety of a radiopharmaceutical candidate. It involves studying the dose-response relationship, determining the exposure levels required for a nuclear imaging agent and for the efficacy of a radiotherapeutic agent, and identifying potential radiotoxic effects at higher doses. Dosimetry estimates the absorbed dose over time for organs and tissues of interest setting the limits of the suggested administered radioactive dose and minimize the risk of adverse reactions due to radiotoxicity. The radiation dosimetry assessment is a practice which is continuous at the different clinical stages also.
By incorporating dosimetric principles, researchers can optimize drug formulations, establish safe dosage ranges, and improve the overall success rate of drug development programs.
Oncodesign Services offers specific studies for novel pharmacoimaging and molecular radiotherapy targets including dosimetry to support your drug development
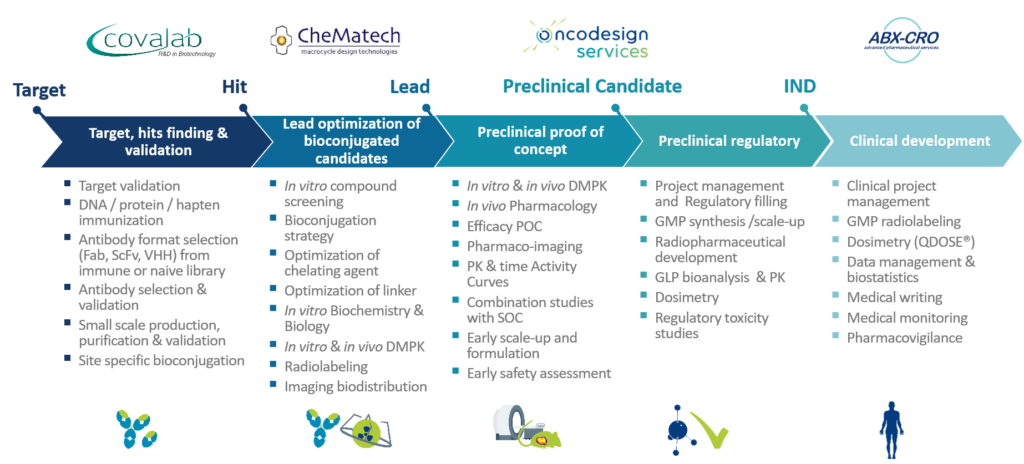
Oncodesign Services offers stand-alone or integrated development package for novel pharmaco-imaging and molecular radiotherapy agents. This offer provides biologics engineering, research-grade and cGMP chelators, bioconjugation and in-life modeling in- as well as preclinical dosimetry assessment and radiopharmaceutical clinical trial design and support with privileged partners.
We use QDOSE® software which gives access to:
- 2D and 3D coregistration
- ROI/VOI drawing and segmentation
- Curve fitting for time activity curves (TACs)
- Calculation of absorbed dose in tissues and organs
- Calculation of effective dose
- Comparison of estimates for different workflows
Discover DRIVE-MRT: A premium “nuclear medicine” solution in Molecular Radiotherapy
DRIVE-MRT is part of our continuous improvement in the integrated drug discovery support. Oncodesign Services and its strategic partners Covalab, CheMatech, and ABX-CRO are applying their expertise in the rationalization, design, and optimization of targeted radiopharmaceutical agents.